Abstract
Introduction and Objective
The costs of the insulin analogue (insulin glargine) have been growing appreciably in the State of Minas Gerais in Brazil, averaging 291 % per year in recent years. This growth has been driven by an increasing number of successful law suits and a 536 % price difference between insulin glargine and neutral protamine Hagedorn (NPH) insulin. One potential way to address this is to undertake a systematic review assessing the efficacy and safety of insulin glargine analogue compared with NPH insulin in patients with type 1 diabetes mellitus (T1DM), and, as a result, provide published data to support future recommended activities by the State of Minas Gerais. These could include maintaining it on the list of the Public Health System (SUS) provided there is a price reduction. Alternatively, the review could provide potential arguments to defend against future law suits should the authorities decide to delist insulin glargine.
Methods
A systematic review of published studies researching the effectiveness of insulin glargine in patients with T1DM between January 1970 and July 2009 in MEDLINE (PubMed), the Latin American and Caribbean Centre on Health Sciences Information, the Cochrane Controlled Trials Databases and the National Health Service Centre for Reviews and Dissemination. Inclusion criteria included insulin glargine on its own or combined with other insulin formulations. Only randomised controlled clinical trials were included. Initially, the titles of all studies were assessed by two independent reviewers before being potentially discarded, with the quality of papers assessed using a modified Jadad scale. The outcome measures included blood levels of glycated haemoglobin, episodes of hypoglycaemia, adverse effects and the reduction of microvascular and macrovascular end-organ complications of T1DM.
Results
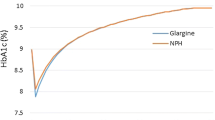
Out of 803 studies found in the selected databases, only eight trials met the inclusion criteria. Most of the studies were of poor methodological quality or had a high risk of bias, with a mean score of 2.125 on the Jadad scale. No study could be classified as double-blind, and only one study documented the increased efficacy of insulin glargine in relation to both glycaemic control and hypoglycaemic episodes. Typically, there was no significant difference between insulin glargine and NPH insulins.
Conclusions
This systematic review showed no therapeutic benefit of insulin glargine over other insulin formulations studied when analysing together glycaemic control and the frequency and severity of hypoglycaemia. We therefore recommend to the State Authority to delist insulin glargine or renegotiate a price reduction with the manufacturer. This systematic review provides support for this decision as well as documentation to combat potential law suits if discussions are unsatisfactory.


Similar content being viewed by others
Notes
Data from the Integrated System of Pharmaceutical Assistance Management of the SES/MG in December 2011.
Data from the Direction of High-Cost Medicines (SES/MG) in February 2012. $US1 = Brazilian real (BRL) 1.72.
References
Godman B, Bennie M, Baumgärtel C, Sović Brkičić L, et al. Essential to increase the use of generics in Europe to maintain comprehensive healthcare? Farmecon Health Econ Ther Pathw. 2012;13(Suppl 3):5–20.
Godman B, Abuelkhair M, Vitry A, Abdu S, et al. Payers endorse generics to enhance prescribing efficiency; impact and future implications, a case history approach. GABI. 2012;1(2):69–83.
Wettermark B, Godman B, Andersson K, Gustafsson LL, Haycox A, Bertele V. Recent national and regional drug reforms in Sweden—implications for pharmaceutical companies in Europe. Pharmacoeconomics. 2008;26:537–50.
Coma A, Zara C, Godman B, Augusta A, Diogenes E, Wettermark B, Haycox A. Policies to enhance the efficiency of prescribing in the Spanish Catalan Region: impact and future direction. Expert Rev Pharmacoecon Outcomes Res. 2009;9:569–81.
Godman B, Wettermark B, Hoffman M, Andersson K, Haycox A, Gustafsson LL. Multifaceted national and regional drug reforms and initiatives in ambulatory care in Sweden; global relevance. Expert Rev Pharmacoecon Outcomes Res. 2009;9:65–83.
Sermet C, Andrieu V, Godman B, Van Ganse E, et al. Ongoing pharmaceutical reforms in France: implications for key stakeholder groups. Appl Health Econ Health Policy. 2010;8:7–24.
Garattini S, Bertele V, Godman B, Haycox A, Wettermark B, Gustafsson LL. Enhancing the rational use of new medicines across European healthcare systems—a position paper. Eur J Clin Pharmacol. 2008;64:1137–8.
Wettermark B, Godman B, Neovius M, Hedberg N, et al. Initial effects of a reimbursement restriction to improve the cost-effectiveness of antihypertensive treatment. Health Policy. 2010;94:221–9.
Cymbalta (duloxetine) receives restricted reimbursement. TLV Sweden. TLV decision duloxetine. http://www.tlv.se/Upload/Genomgangen/100615-tlv-interim-decision-cymbalta.pdf. Accessed 20 Apr 2013.
Pettersson B, Hoffmann M, Wändell P P, Levin L-A. Utilization and costs of lipid modifying therapies following health technology assessment for the new reimbursement scheme in Sweden. Health Policy. 2012;104:84–91.
Godman B, Persson M, Miranda J, et al. Changes in the utilisation of venlafaxine after the introduction of generics in Sweden. Appl Health Econ Health Policy. 2013;11(4):383–93.
Godman B, Schwabe U, Selke G, Wettermark B. Update of recent reforms in Germany to enhance the quality and efficiency of prescribing of proton pump inhibitors and lipid lowering drugs. Pharmacoeconomics. 2009;27:435–8.
Institut für qualität und Wirtschaftlichkeit im Gesundheitswesen [Institute of Quality and Efficiency in Health Care]. Long-acting insulin analogues in the treatment of diabetes mellitus type 1. 2010. http://www.iqwig.de/download/A05-01_Executive-Summary_Long-acting_insulin_analogues_in_diabetes_mellitus_type_1.pdf. Accessed Sep 2010.
Holden SE, Poole CD, Morgan CL, et al. Evaluation of the incremental cost to the National Health Service of prescribing analogue insulin. BMJ Open. 2011;1:e000258. doi:10.1136/bmjopen-2011-000258.
Institute for Quality and Efficiency in Health Care—IQWIG. Long-acting insulin analogues in the treatment of diabetes mellitus type 2: final report; commission A05e03 (in German). 2008. https://www.iqwig.de/download/A05-03_Executive_summary_Long_acting_insulin_analogues_in_the_treatment_of_diabetes_mellitus_type_2.pdf. Accessed 17 May 2013.
Institute for Quality and Efficiency in Health Care—IQWIG. Long acting insulins for the treatment of diabetes mellitus type 1—documentation. 2008. https://www.iqwig.de/download/A05-01_Dokumentation_und_Wuerdigung_der_Stellungnahmen_zum_Berichtsplan_V_1_0.pdf. Accessed 17 May 2013.
Schwartzkopff F. Lilly, Novo cut insulin prices for German customers. 2007. http://www.bloomberg.com/apps/news?pid=newsarchive&sid=aqvban0jOndE. Accessed 17 May 2013.
Vardi M, Eyal J, Asaph N, Haim B. Intermediate acting versus long acting insulin for type 1 diabetes mellitus. Cochrane Database Syst Rev. 2008;(3):CD006297.
Horvath K, Jeitler K, Berghold A, Ebrahim SH, Gratzer TW, Plank J, Kaiser T, Pieber TR, Siebenhofer A. Long-acting insulin analogues versus NPH insulin (human isophane insulin) for type 2 diabetes mellitus. Cochrane Database Syst Rev. 2007;(2):Art. No.: CD005613. doi:10.1002/14651858.CD005613.pub3.
National Institute for Clinical Excellence. Guidance on the use of long-acting insulin analogues for the treatment of diabetes insulin glargine. London: NICE; 2002 (technology appraisal 53). http://www.nice.org.uk/nicemedia/live/11482/32518/32518.pdf. Accessed 17 May 2013.
Moodie P. More from PHARMAC on long-acting insulin analogues: insulin glargine now funded. N Z Med J. 2006;119:118.
Singh SR, Ahmad F, Lal A, Yu C, Bai Z, Bennett H. Efficacy and safety of insulin analogues for the management of diabetes mellitus: a meta-analysis. CMAJ. 2009;180(4):385–97.
Monami M, Marchionni N, Mannucci E. Long-acting insulin analogues vs. NPH human insulin in type 1 diabetes: a meta-analysis. Diabetes Obes Metab. 2009;11(4):372–8.
MINAS GERAIS. Resolução SES/MG No. 1953 de 20 de julho de 2009. http://www.saude.mg.gov.br/images/documentos/resolucao_1953.pdf. Accessed 8 June 2013.
Godman B, Bucsics A, Burkhardt T, Haycox A, Seyfried H, Wieninger P. Insight into recent reforms and initiatives in Austria: implications for key stakeholders. Expert Rev Pharmacoecon Outcomes Res. 2008;8:357–71.
Malmström E, Godman B, Diogene E, Baumgärtel C, Bennie B, et al. Dabigatran—a case history demonstrating the need for comprehensive approaches to optimise the use of new drugs. Front Pharmacol. 2013;4:1–19. doi:10.3389/fphar.2013.00039.
Godman B, Gustafsson LL. A new reimbursement systems for innovative pharmaceuticals combining value-based and free market pricing. Appl Health Econ Health Policy. 2013;11:79–82.
Neto O, Acurcio F, de Ávila M, et al. Doctors, lawyers and pharmaceutical industry on health lawsuits in Minas Gerais, Southeastern Brazil. Rev Saúde Pública. http://www.scielo.br/rsp.
Cohen D, Carter P. How small changes led to big profits for insulin manufacturers. BMJ. 2010;341:c7139.
Machado MAA, Acurcio FA, Brandão CMR, Faleiros DR, Guerra Júnior AA, Cherchiglia ML, Andrade EIG. Judicialização do acesso a medicamentos no Estado de Minas Gerais, Brasil (Judicialisation of access to medicines in the State of Minas Gerais, Brazil). Rev Saúde Pública. 2011;45(3):590–8.
Rede Brasileira de Avaliação de Tecnologia em Saúde (Brazilian Network for Health Technology Assessment): Boletim Brasileiro de Avaliação de Tecnologias em Saúde (Brazilian Bulletin of Health Technology Assessment). Insulina Glargina e Insulina Detemir no Controle da Diabetes Mellitus Tipo 1 (Glargine insulin and detemir insulin: the control of type 1 diabetes mellitus). 2010. http://bvsms.saude.gov.br/bvs/ct/pdf/brats2010_n13.pdf. Accessed 5 June 2013.
Hemkens LG, Grouven U, Bender R, Günster C, Gutschmidt S, Selke GW, et al. Risk of malignancies in patients with diabetes treated with human insulin or insulin analogues: a cohort study. Diabetologia. 2009;52(9):1732–44.
Jonasson JM, Ljung R, Talbäck M, Haglund B, Gudbjörnsdòttir S, Steineck G. Insulin glargine use and short-term incidence of malignancies—a population-based follow-up study in Sweden. Diabetologia. 2009;52(9):1745–54.
Plank J, Siebenhofer A, Berghold A, Jeitler K, Horvath K, Mrak P, et al. Systematic review and meta-analysis of short-acting insulin analogues in patients with diabetes mellitus. Arch Intern Med. 2005;165(12):1325–444.
Woodroffe R, Yao GL, Meads C, Bayliss S, Ready A, Raftery J, et al. Clinical and cost-effectiveness of newer immunosuppressive regimens in renal transplantation: a systematic review and modelling study. Health Technol Assess. 2005;9(21):1–179, iii–iv.
White NH, Chase HP, Arslanian S, Tamborlane WV. Comparison of glycaemic variability associated with insulin glargine and intermediate-acting insulin when used as the basal component of multiple daily. Diabetes Care. 2009;32(3):387–93.
Rosenstock J, Park G, Zimmerman J. Basal insulin glargine (HOE 901) versus NPH insulin in patients with type 1 diabetes on multiple daily insulin regimens. U.S. Insulin Glargine (HOE 901) Type 1. Diabetes Care. 2000;23(8):1137–42.
Pieber TR, Eugene-Jolchine I, Derobert E. Efficacy and safety of HOE 901 versus NPH insulin in patients with type 1 diabetes. The European Study Group of HOE 901 in type 1 diabetes. Diabetes Care. 2000;23(2):157–62.
Doyle EA, Weinzimer SA, Steffen AT, Ahern JA, Vincent M, Tamborlane WV. A randomized, prospective trial comparing the efficacy of continuous subcutaneous insulin infusion with multiple daily injections using insulin glargine. Diabetes Care. 2004;27(7):1554–8.
Raskin P, Klaff L, Bergenstal R, Halle JP, Donley D, Mecca T. A 16-week comparison of the novel insulin analog insulin glargine (HOE 901) and NPH human insulin used with insulin lispro in patients with type 1 diabetes. Diabetes Care. 2000;23(11):1666–71.
Chatterjee S, Jarvis-Kay J, Rengarajan T, Lawrence IG, McNally PG, Davies MJ. Glargine versus NPH insulin: efficacy in comparison with insulin aspart in a basal bolus regimen in type 1 diabetes—the glargine and aspart study (GLASS). Diabetes Res Clin Pract. 2007;77(2):215–22.
Schober E, Schoenle E, Van Dyk J, Wernicke-Panten K. Comparative trial between insulin glargine and NPH insulin in children and adolescents with type 1 diabetes mellitus. J Pediatr Endocrinol Metab. 2002;15(4):369–76.
Chase HP, Arslanian S, Neil HW, Tamborlane WV. Insulin glargine versus intermediate-acting insulin as the basal component of multiple daily injection regimens for adolescents with type 1 diabetes mellitus. J Pediatr. 2008;153:547–53.
Scottish Medicines Consortium. Insulin glargine 100 units/ml solution for injection in a vial, cartridge, pre-filled pen—No: 860/13. 2013. http://www.scottishmedicines.org.uk/files/advice/insulin_glargine_Lantus_Abbreviated_FINAL_March_2013_for_website.pdf. Accessed 17 May 2013.
Area Integral de Salut, Barcelona. Protocol de Tractament de la Diabetis Tipus 2. http://www.gencat.cat/salut/botss/html/ca/dir3457/daibetis_tipus2.pdf. Accessed 17 May 2013.
Bennie M, Godman B, Bishop I, Campbell S. Multiple initiatives continue to enhance the prescribing efficiency for the proton pump inhibitors and statins in Scotland. Expert Rev Pharmacoecon Outcomes Res. 2012;12:125–30.
World Health Organization: 18th Expert Committee on the selection and use of Essential Medicines. Review of the evidence comparing insulin (human or animal) with analogue insulins. 2011. http://www.who.int/selection_medicines/committees/expert/18/applications/Insulin_review.pdf. Accessed 3 June 2013.
Zib I, Raskin P. Novel insulin analogues and its mitogenic potential. Diabetes Obes Metab. 2006;8:611–20.
Currie CJ, Poole CD, Gale EAM. The influence of glucose-lowering therapies on cancer risk in type 2 diabetes. Diabetologia. 2009;52:1766–77.
Smith U, Gale EAM. Does diabetes therapy influence the risk of cancer? Diabetologia. 2009;52:1699–708.
Gill GV, Yudkin JS, Keen H, Beran D. The insulin dilemma in resource-limited countries. A way forward? Diabetologia. 2010;54(1):19–24.
Godman B, Sakshaug S, Berg C, Wettermark B, Haycox A. Combination of prescribing restrictions and policies to engineer low prices to reduce reimbursement costs. Expert Rev Pharmacoecon Outcomes Res. 2011;11:121–9.
Godman B, Malmstrom RE, Bennie M, Sakshaug S, Burkhardt T, et al. Prescribing restrictions—a necessary strategy among some European countries to enhance future prescribing efficiency? Rev Health Care. 2012;3:5–16.
Bucsics A, Godman B, Burkhardt T, et al. Influence of lifting prescribing restrictions for losartan on subsequent sartan utilisation patterns in Austria: implications for other countries. Expert Rev Pharmacoecon Outcomes Res. 2012;12:809–19.
Adamski J, Godman B, Ofierska-Sujkowska G, et al. Review of risk sharing schemes for pharmaceuticals: considerations, critical evaluation and recommendations for European payers. BMC Health Serv Res. 2010;10:153. doi:10.1186/1472-6963-10-153.
Cheema PK, Gavura S, Migus M, Godman B, Yeung L, Trudeau M. International variability in the reimbursement of cancer drugs by publically funded drug programs. Curr Oncol. 2012;19:165–176. http://dx.doi.org/10.3747/co.19.946.
Shirk RC, Godman B. Developing risk sharing arrangements—potential for Brazil and implications. Value Health. 2011;14(7):A557.
Godman B, Paterson K, Malmstrom R, et al. Improving the managed entry of new medicines: sharing experiences across Europe. Expert Rev Pharmacoecon Outcomes Res. 2012;12:439–41.
Acknowledgments
The authors thank the Minas Gerais State Health Secretariat and the National Council for Scientific and Technological Development (CNPq) for funding this study. We also thank the Federal University of Minas Gerais for its support.
The authors declare that they have no conflicts of interest that may have influenced this study.
No writing assistance was provided in the production of the manuscript.
Author contributions
Ana Luísa Caires de Souza, Francisco de Assis Acurcio, Augusto Afonso Guerra Júnior, Renata Cristina Rezende Macedo do Nascimento and Leonardo Maurício Diniz contributed equally to both the conception/design and analysis/interpretation of the project and the writing of the paper. Brian Godman provided assistance with the study design and critiqued successive drafts to provide a global perspective.
Ana Luísa Caires de Souza is the guarantor of the overall content of the paper.
Author information
Authors and Affiliations
Corresponding author
Appendix
Rights and permissions
About this article
Cite this article
Caires de Souza, A.L., Acurcio, F.A., Guerra Júnior, A.A. et al. Insulin Glargine in a Brazilian State: Should the Government Disinvest? An Assessment Based on a Systematic Review. Appl Health Econ Health Policy 12, 19–32 (2014). https://doi.org/10.1007/s40258-013-0073-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40258-013-0073-6