Abstract
Isotretinoin was formulated in novel microemulsion-based gel formulation with the aim of improving its solubility, skin tolerability, therapeutic efficacy, skin-targeting efficiency and patient compliance. Microemulsion was formulated by the spontaneous microemulsification method using 8 % isopropyl myristate, 24 % Labrasol, 8 % plurol oleique and 60 % water as an external phase. All plain and isotretinoin-loaded microemulsions were clear and showed physicochemical parameters for the desired topical delivery and stability. The permeation profiles of isotretinoin through rat skin from selected microemulsion formulation followed zero-order kinetics. Microemulsion-based gel was prepared by incorporating Carbopol®971 in optimized microemulsion formulation having suitable skin permeation rate and skin uptake. Microemulsion-based gel showed desired physicochemical parameters and demonstrated advantage over marketed formulation in improving the skin tolerability of isotretinoin, indicating its potential in improving topical delivery of isotretinoin. The developed microemulsion-based gel may be a potential drug delivery vehicle for targeted topical delivery of isotretinoin in the treatment of acne.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acne, a cutaneous disorder of multifactorial origin, is a disease whose cause and severity depend on the relationship between hormones, keratinization, sebum and bacteria. The objectives of acne therapy usually include controlling acne lesions, preventing scarring and minimizing morbidity (Russell 2000; Zaenglein 2008).
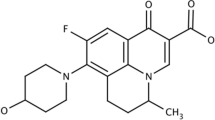
Isotretinoin (ITTN), a derivative of retinoic acid (13-cis-retinoic acid), is the most effective compound with potential to suppress acne over the long term (Zaenglein 2008; Patel et al. 2011a). It appears to derive its effectiveness from increased production of the antimicrobial protein neutrophil gelatinase-associated lipocalin in the skin reducing sebum levels and in turn reducing levels of Propionibacterium acnes (P. acnes). An ongoing trial in patients with antibiotic-resistant P. acnes indicates that ITTN is highly effective; treatment of P. acnes may well become a new indication for this drug (Zaenglein 2008). So, ITTN, reducing the growth of P.acnes in a secondary manner, was selected for further study. However despite these interesting features, extremely low solubility limits ITTN incorporation into an acceptable vehicle and its tolerability results in either discontinuation of treatment or poor compliance in patients (Zaenglein 2008; Patel et al. 2011a). Therefore, it is prudent to improve the topical delivery and reduce adverse effects of ITTN using an appropriate carrier having the desired skin-targeting properties that may reduce the adverse reactions substantially and improve users’ compliance to the novel regimen.
The conventional anti-acne formulations could be less efficacious due to insignificant skin penetration properties. In comparison, the novel drug delivery systems of recently available anti-acne formulations have potential to reduce the adverse effects without reducing the efficacy. Microemulsion (ME) offers a powerful way for drug delivery as colloidal drug carrier, due to its versatility and favorable advantages (Bachhav and Patravale 2009; Djordjevic 2004; Patel et al. 2009, 2011b, 2013a, b, c). The application of ME systems to skin distributes the anti-acne agent(s) steadily and reduce irritation. These systems have also been shown to promote follicular targeting and thus create high local concentrations of the active compound in the pilosebaceous unit and efficient penetration in hair follicles (Zaenglein 2008; Patel et al. 2011a). Thus, MEs could play a pivotal role in improving topical delivery of anti-acne agents by enhancing their dermal localization with concomitant reduction in their adverse effects. ME-based gels (MBG) are known to provide faster drug release than conventional formulations (Bachhav and Patravale 2009). A ME-based gel was thus developed and evaluated using a suitable polymer that is capable of modifying the rheological behavior.
To find out innovative ways for administering ITTN and assuaging their disadvantages, the present study investigated the development of ME-based gel containing ITTN. It was hypothesized that the oily core present around ITTN may result in the reduction of irritation when presented in the form of ME. The combination of effects, viz. equal and/or enhanced effectiveness, reduction in adverse reactions and regimen adherence may provide a better platform of novel drug delivery with improved therapeutic efficacy. Thus, the present study focuses on novel formulation consideration, characterization and evaluation of skin-targeting efficiency of ITTN when loaded with MBG.
Materials and methods
Materials
ITTN was procured as gratis samples from Astron Research Ltd. (Ahmedabad, India). Labrasol, plurol oleique (Gattefosse, Saint-Priest, France), Carbopol 980 NF, Carbopol 971P NF, Carbopol 974P NF, (Lubrizol Advanced Materials India Pvt. Ltd., Mumbai, India), Methocel E50 LV premium hydroxypropyl methylcellulose EP grade and Methocel KM premium hydroxypropyl methylcellulose (Colorcon Asia Pvt. Ltd., Goa, India) were procured on gratis. Isopropyl myristate was purchased from National Chemicals (Vadodara, India). Ethanol was purchased from Baroda Chemical Ind. Ltd. (Dabhoi, India). Double distilled water was used throughout the study. All other chemicals and solvents were of analytical reagent grade and were used as received without further purification.
Methods
Solubility determination
The solubility of ITTN was determined in various oils, emulsifiers, and co-emulsifiers. In this experiment, excess amount of ITTN was added in 1 mL of each excipient and mixed thoroughly using vortexer at ambient temperature (Patel et al. 2009). The mixed samples were kept for 72 h to allow equilibration. Then the samples were centrifuged at 5000×g for 30 min to separate the undissolved drug. Supernatants were diluted and analyzed by the HPTLC method (Patel et al. 2011a) to estimate the concentration of soluble ITTN. Excipients in which ITTN showed good or excellent solubility were chosen for preparing MEs.
Construction of phase diagrams
The pseudo-ternary phase diagrams were constructed using water titration method at ambient temperature. Three phase diagrams were constructed with the 1:1, 2:1 and 3:1 weight ratios of emulsifier/co-emulsifier mixture (Emix) of Labrasol (LAB) and plurol oleique (PO), respectively. For each phase diagram at a specific emulsifier/co-emulsifier mixing ratio (Km), the ratio of isopropyl myristate (IPM) to the Emix was varied as 1:9, 2:8, 3:7, 4:6, 5:5, 6:4, 7:3, 8:2 and 9:1. Spontaneous formation of ME was evaluated by the addition of a known amount of water at once to a known amount of oil, emulsifier and co-emulsifier with controlled stirring. The ease of formation of clear ME was taken as the criteria of spontaneity of microemulsification (Patel et al. 2009, 2011b, 2013a, b, c).
Preparation of microemulsions
Six potential ME formulations, which were different from each other by water volume fraction, were selected and formulated at Km 3:1 by the spontaneous microemulsification method. A calculated amount of ITTN (0.05 % w/w) was added to the oily phase of ME and magnetically stirred until dissolved, followed by addition of Emix in a fixed proportion to produce a clear mixture. Then, a defined proportion of water was added and stirred to produce clear ME containing ITTN (Patel et al. 2013c).
Preparation of microemulsion-based gel
Carbopol® 971P NF was selected as the gel matrix to prepare ME-based gel (MBG). It was entirely swollen upon slow mixing in the water and its pH was adjusted by adding 50 % w/w triethanolamine (TEA). MBG was obtained by mixing the swollen gel in water with the oily phase (Bachhav and Patravale 2009; Chen et al. 2007).
Characterization of formulations
The average droplet size and polydispersity index (PDI) of ME were measured by photon correlation spectroscopy (PCS) with in-built Zetasizer (Nano ZS, Malvern Instruments, UK) at 633 nm. Helium–neon gas laser having intensity of 4mW was the light source. The droplet size was calculated using Stokes–Einstein relationship by Zetasizer Software. Electrophoretic mobility (µm/s) was measured using small volume disposable zeta cell and converted to zeta potential by in-built software using Helmholtz–Smoluchowski equation. All determinations were made in triplicate. TEM was used to characterize the microstructure of ITTN-loaded ME. The TEM images were obtained using a Tecnai G2 20 TEM (Philips, Holland). ITTN content in the formulation was assayed using an HPTLC (Patel et al. 2011a). The percent transmittance of the formulation was measured using a colorimeter (Digital Colorimeter, D-801, Photocon, India) at 570–590 nm. The isotropic nature of the ME formulations was verified using cross-polarized light microscopy (Polarizing Microscope RPL-55 Series, Radical Instruments, India). The pH value of the formulation was determined using a digital pH meter (HI 98107, Hanna Instruments, India) which was standardized using pH 4 and 7 buffers before use. The electric conductivity of ME was measured using a conductivity meter (Equip-Tronics, EQ—664, Mumbai, India) equipped with an in-built magnetic stirrer. The viscosity of ME was measured using a Brookfield Viscometer LVDV-IIIU (Brookfield Engineering LABS, Stoughton, USA) with spindle SC 18 at 100 rpm using an interval of 30 s. A rheological study of the MBG was performed using a viscometer with Helipath stand (Patel et al. 2009, 2011b, 2013a, b, c).
Determination of spreadability of microemulsion-based gel
The spreadability of the gel was determined by placing 0.5 g gel within a pre-marked circle of 1 cm diameter on a glass plate over which a second glass plate was placed. A weight of 500 g was allowed to rest on the upper glass plate for 5 min. The increase in the diameter due to spreading of the gels was noted and the mean diameter was taken by repeating the experiment three times (Bachhav and Patravale 2009).
In vitro skin permeation study
The in vitro skin permeation study was carried out under the guidelines proposed by the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) and all the study protocols were approved by the Local Institutional Animal Ethics Committee (IAEC). The abdominal skins obtained from male Wistar rats weighing 230 ± 20 g (age, 6–8 weeks) were used for in vitro permeation experiments of the prepared formulations. After hair was shaved carefully with an electric clipper, the skin was excised from the abdominal region of each killed rat and the subcutaneous fat and other extraneous tissues were removed without damaging the epidermal surface. The excised rat skins were rinsed with physiological saline and the thickness of each skin tissue was kept almost similar (Patel et al. 2009, 2011b).
The excised skins were then placed in a refrigerator at 4 °C and then used for permeation experiments. The skin membranes were first hydrated for 30 min in buffer solution (pH 7.4) at room temperature to remove extraneous debris and leachable enzymes. The permeation experiments were performed using Franz diffusion cells fitted with excised rat skins, such that the dermal side of the skin was exposed to the receptor fluid and the stratum corneum remained in contact with the donor compartment. The effective permeation area was 3.14 cm2 (20 mm-diameter orifice), and the receptor compartment was filled with 12 mL of physiological saline:ethanol (7:3 v/v) for ensuring pseudo-sink conditions by increasing the solubility of ITTN in the receiving phase. The temperature of the receiver chamber containing 12 mL of receptor media was controlled at 32 ± 1 °C using circulating equibath (Model 8506, Medica Instrument Mfg. CO, Mumbai, India). The diffusion medium was continuously stirred with a Teflon-coated magnetic bar at a constant rate, in a way that the rat skin surface just flushed the diffusion fluid. The formulation (1 g) was gently placed in a donor chamber and then the diffusion cells were covered with an aluminum foil to prevent light exposure. At 1, 2, 4, 6, 8, 10 and 12 h, aliquots of 1 mL sample were withdrawn from the receptor compartment and replaced immediately with an equivalent volume of receptor fluid. The concentration of ITTN in receptor fluid was analyzed using the HPTLC method (Patel et al. 2011a). Each experiment was performed in triplicate. Cumulative corrections were made to obtain the total amount of ITTN permeated at each time interval.
The permeation rate was determined using the equation, J ss = 1/A(dQ/dt)ss = C 0 K p , where J ss is the permeation rate at steady state (μg/cm2/h), A the effective diffusion area (cm2), (dQ/dt)ss the amount of drug that permeated through the skin per unit time at steady state (μg/h), C 0 the applied drug concentration in the donor compartment (μg/mL) and K p the permeability coefficient (cm/h). The average cumulative amount of drug that permeated per unit surface area of the skin was plotted versus time. The permeation rate of ITTN at steady state (J ss , μg/cm2/h) through rat skin was calculated from the slope of the linear portion of the cumulative amount that permeated through the rat skins per unit area versus time plot. To obtain the permeability coefficient K p (cm/h), the following equation was used: K p = J ss /C 0 (Patel et al. 2009, 2011b).
Stability studies
The stability of ME formulations was studied through various parameters such as clarity, phase separation observation, droplet size determinations, viscosity measurements, conductivity measurements, pH and zeta potential determinations for 6 months at room temperature. To estimate metastable systems, the selected ME vehicles were centrifuged (Remi Lab, Mumbai) at 5000×g for 30 min. Besides the physicochemical properties, the chemical stability of the investigated drug in the vehicle plays a major role. Therefore, the drug content was analyzed at defined time intervals during the observation period of 6 months. During the observation period, the formulations were stored at room temperature in tubes to simulate patient usage conditions. During stability studies, the samples were taken at fixed time intervals (Patel et al. 2011b).
Infrared study
The infrared (IR) spectra of ITTN, unloaded ME, optimized ITTN-ME and ITTN-MBG were taken using an IR spectrophotometer (Spectrum GX FT-IR, Perkin Elmer, Norwalk, CT). The unloaded ME, ITTN-ME and ITTN-MBG were spread as a thin layer on potassium bromide cell and then scanned between 4000 and 400 cm−1. The resulting IR spectra of ITTN and plain ME were then compared with ITTN-ME and ITTN-MBG to detect any possible interaction between the drug and different components used (Patel et al. 2013c).
Skin retention study
Skin retention study was performed to analyze the content of the drug in the skin. At the end of the in vitro skin permeation study, the skin samples were washed with water and methanol on both sides and carefully dried. Then a defined amount of methanol was added to each piece of skin. The samples were vortexed for 10 min to extract its drug content and stirred overnight. The samples were analyzed by HPTLC (Patel et al. 2011a) after centrifugation (Bachhav and Patravale 2009).
Histopathological investigation
The rat abdominal skin was mounted on Franz diffusion cell. The optimized ME, MBG (1 g) and marketed gel were applied similar to method for permeation study and the effects were compared against control. A piece of fresh excised untreated skin sample was used as control. The skin was fixed in 10 % neutral formalin for 24 h and then cut vertically against the surface at the central region (4 mm width). Each section was dehydrated using graded solutions of ethanol and then embedded in paraffin wax. Tissues were divided into small pieces and stained with hematoxylin and eosin. The sections were observed under 100× magnifications and photographed (Patel et al. 2009, 2011b, 2013a, b, c).
Skin irritation testing (Draize patch test)
The irritation potential of the ME-based ITTN gel in comparison to marketed ITTN gel was evaluated by carrying out the Draize patch test on rabbits. Animal care and handling throughout the experimental procedure were performed in accordance with the CPCSEA guidelines. White New Zealand rabbits weighing 2.5–3 kg were acclimatized before the beginning of the study.
Animals were divided into four groups (n = 3) as follows:
Group 1: no application (control).
Group 2: marketed formulation (Sotret® gel containing 0.05 % w/w ITTN, Ranbaxy).
Group 3: MBG without ITTN (placebo gel).
Group 4: MBG containing ITTN (0.05 %, w/w).
The back of the rabbits were clipped free of hair 24 h prior to the application of the formulations. 0.5 g formulations were applied on the hair-free skin of rabbits by uniform spreading within the area of 4 cm2. The skin was observed for any visible change such as erythema (redness) at 24, 48 and 72 h after the application of various formulations. The mean erythema scores were recorded (ranging from 0 to 4) depending on the degree of erythema as follows: no erythema = 0, slight erythema (barely perceptible-light pink) = 1, moderate erythema (dark pink) = 2, moderate to severe erythema (light red) = 3 and severe erythema (extreme redness) = 4 (Draize et al. 1944; Shah et al. 2007).
Statistical analysis
All the skin permeation studies were carried out in triplicate and the data were expressed as the mean value ± SD. Statistical data were analyzed by one-way analysis of variance (ANOVA). A multiple comparison test was used to compare different formulations and P < 0.05 was considered to be significant.
Results and discussion
Development and evaluation of microemulsion
The solubility of ITTN in various oils, emulsifiers and co-emulsifiers was analyzed to screen for components of ME. The solubility of ITTN was found to be highest in IPM (42.92 ± 3.97 mg/gm), a low molecular volume fatty acid ester and one of the most often used oils along with long chain triglycerides and medium chain triglycerides for ME (Subramanian et al. 2005). It has been reported that the large solubilizing capacity of ME leads to larger concentration gradient toward the skin, providing superior dermal flux, for which high solubility of ITTN in oily phase can be an added advantage to increase it in ME. Also, literature reveals that IPM acts as a chemical enhancer by disordering the prearranged lipids of the horny layer. To study the influence of oil on ME characteristics and penetration-enhancing ability, IPM was chosen for the preparation of ME-containing ITTN (Patel et al. 2009, 2011b, 2013a, b, c; Subramanian et al. 2005).
The solubility of ITTN was determined in different emulsifiers to find a suitable emulsifier for the preparation of ME. The highest solubility of ITTN was found in LAB (86.81 ± 4.82 mg/gm) which is a non-ionic emulsifier used as a pharmaceutical excipient for the solubilization of hydrophobic drugs. There have been several studies involving low-irritant LAB-based MEs as drug delivery vehicles for topical application (Alvarez-Figueroa and Blanco-M´endez 2001; Djekic and Primorac 2008). Also, it has been reported that LAB-based systems incorporating fatty acid esters in an oil phase have a large ME region in their phase diagrams. To facilitate the formulation of LAB-based MEs, PO has been used as co-emulsifier (Fanun 2008). It has also been reported that solubilization of water is easier in systems containing LAB as emulsifier and PO as a co-emulsifier. The highest solubility of ITTN was found in PO (10.04 ± 3.54 mg/gm) among the co-emulsifiers studied. From these results, IPM, LAB and PO were subsequently used as the oil phase, emulsifier and co-emulsifier for the formulation of MEs containing ITTN in this study.
The pseudoternary phase diagrams of the system of IPM, LAB, PO and water were constructed to find out the existence range of MEs. The phase diagrams are represented in Fig. 1. The phase boundary was determined by observing the changes of the sample appearance from turbid to transparent or from transparent to turbid based on visual identification. The isotropic and low-viscosity region is presented in the phase diagram as the one-phase ME region (1Φ) and the remainder of the phase diagram represents the turbid region, designated as multiphase (MΦ) conventional emulsions. From a microstructural point of view, MEs may sometimes be envisaged as emulsifier aggregrates of water dispersed (solubilized) in oil (w/o) or oil dispersed in water (o/w). When the ME region extends from an oil-rich region (i.e., w/o ME) to a water-rich region (i.e., o/w ME), the transition between these two regions passes through a bicontinuous isotropic region (Fanun 2008). The area of ME isotropic region changed slightly in size with Km in the order of: 3:1 > 2:1 > 1:1. The phase study revealed that the maximum solubilization of water was achieved in ME systems when Km was 3:1 and so it was fixed at 3:1 for further studies.
The pseudoternary phase diagrams of the isopropyl myristate, Labrasol/plurol oleique (Emix), water at the 1:1 (X), 2:1 (Y) and 3:1 (Z) weight ratios of Emix (Km) at ambient temperature. The one-phase region is designated by 1Φ and the multiphase region by MΦ. The dilution line (a, b) at oil/Emix of 0.25 represents the investigated systems’ compositions (I1 to I6)
The maximum percentage of water in the phase diagram with IPM as an oil phase (Fig. 1z) was 86.94 % w/w for the ratio of oil to Emix of 0.11 with the content of Emix at Km = 3:1 of 9.89 % w/w and 76 % w/w for ratio of oil to Emix of 0.25 with content of Emix at Km = 3:1 of 20 % w/w. The obtained results suggested that the maximum solubilization of water (Wmax) was increased with decrease in the amphiphile content in the ratio of oil to Emix in the order of 0.11 > 0.25 > 0.42 > 0.6 > 1 > 1.5 > 2.3 > 9. The Wmax was achieved at a ratio of oil to Emix less than 0.25. IPM was solubilized completely with LAB and the Wmax of IPM/LAB mixture was found to be higher. It was considered that IPM, due to its preferable chemical structure, probably penetrated the interfacial layer and interacted with LAB, increasing its efficiency in solubilizing water. Hence, the obtained results indicated that LAB showed a good efficiency and water-solubilizing capacity in the presence of IPM. These observations were quite consistent with previously reported results (Djekic and Primorac 2008).
Further six potential ME vehicles, I1–I6, for IPM as an oil phase (Table 1) were selected at a ratio of oil to Emix of 0.25 along the dilution line A–B in Fig. 1z. The entire drug-loaded ME formulations containing ITTN were prepared in the same ratios and the proportion of ITTN was kept constant at 0.05 % w/w for all the batches.
The average particle size of the plain MEs and drug-loaded ME were determined. The results of particle size analysis of ME formulation containing IPM as an oil phase are shown in Table 1 for plain MEs and Table 2 for drug-loaded MEs. The unloaded vehicles have a globule size in the range of 22.4–85.9 nm, while drug-loaded vehicles have a globule size in the range of 16.9–72.3 nm. Two explanations, (1) undissolved drug acting as a emulsifier and (2) reduction in emulsifier mobility, are offered in the literature for the decrease in droplet size with the addition of the drug due to deposition of drug particles at the interface of ME (Biruss and Valenta 2008; Djekic and Primorac 2008; Fanun 2008; Kantarci et al. 2007). It has been reported that the lowest conductivity plots extend to the largest droplet radius. The size of the droplet of unloaded ME with low conductivity values was greater than that of the ITTN-loaded formulation with higher conductivity values (Patel et al. 2009, 2011b, 2013a, b, c). All the formulations had droplets in the nano range, which is very well evident from the low PDI values for both unloaded and ITTN-loaded vehicles as described in Tables 1 and 2. Although the PDI values of all formulations were very low, indicating uniformity of droplet size within each formulation, it was least for I6 (0.122 ± 0.012). An emulsifier can stabilize the emulsion, not only just by forming a mechanical barrier, but also by producing an electrical (electrostatic) barrier or surface charge. The electrical surface charge of the droplets is produced by the ionization of interfacial film-forming components. The surface potential and the resulting zeta potential of emulsion droplets will depend on the extent of ionization of emulsifiers. For MEs with non-ionic emulsifier, the zeta potential can be used to analyze the charge of the system (Biruss and Valenta 2008). The zeta potential of plain and ITTN-loaded ME formulations are presented in Tables 1 and 2, respectively. The zeta potential values of all the formulations are less than −30 mv. It can be seen that the ME system is negatively charged and on addition of ITTN, the zeta potential values in the ME did not change significantly (Patel et al. 2013a, b, c). The % T values of plain and ITTN-loaded ME formulations were between 98 and 100 % (Tables 1 and 2). Hence, all the plain and medicated ME formulations were found to be optically clear. The TEM imaging of ITTN-loaded ME formulation is shown in Fig. 2. The TEM images revealed that the particle size was in the nanometric range and that particle had nearly spherical morphology without aggregation which is in accordance with particle size analysis. Figure 3 shows the particle size distribution by intensity of optimized ITTN-loaded ME formulation. The ITTN content of ME formulations was found to be in the range of 99.12–99.98 % w/w of the theoretical value (0.05 % w/w) (Table 2). The samples were examined by ocular inspection in a cross polarizer for sample homogeneity and birefringence. All ME formulations appeared completely dark when observed under cross polarizer which confirmed its optically isotropic nature (Draize et al. 1944; Shah et al. 2007). The rate of diffusion of a drug is dependent on the pH of the formulation. It is one of the factors influencing skin penetration (Patel et al. 2013a, b, c). The pH values for ME formulations were found in the range of 6.28–6.67 (Table 1). The incorporated ITTN did not affect the optical texture of the ME formulations and did not influence the pH values of the ME formulations significantly (Table 2). Electrical conductivity is a structure-sensitive property and there are some studies reported on the systems of non-ionic emulsifiers. Although reported in literature, the conductivity measurement were done without incorporating a small amount of aqueous electrolyte (NaCl) responsible for charge transport due to its effect on phase behavior and structural properties of MEs, resulting in phase separation. The investigated ME formulations containing non-ionic amphiphiles exhibited electroconductive behavior, in spite of its non-ionic nature. Figure 4a displays the influence of water volume fraction (Φw, % w/w) change on the electrical conductivity (σ, µS/cm) of ME formulations. Along with the increase in water content, the values of electrical conductivity increased continuously. The low conductivity values indicate restricted water mobility, while high values suggest that the system undergoes a structural inversion from w/o to o/w MEs. These changes have been attributed to the occurrence of a percolation transition. In the percolation threshold model, the conductivity remains low up to a certain critical volume fraction of water (Φc) as the w/o droplets surrounded by continuous oil phase are isolated from each other, thus contributing very little to electrical conductance. When the volume fraction of water reaches the percolation threshold (Φc = Φp), clusters of some conductive droplets which begin to contact each other are formed. Beyond Φp the conductivity increases sharply and rapidly as the number of clusters increases. This has been attributed to the formation of water cylinders or channels in an oil phase due to the sticky collisions through transient merging of spherical microdroplets of water phase in the w/o ME (Fanun 2008). As shown in Fig. 4a, the electrical conductivity of the oily mixtures containing IPM was almost zero as long as Φw was smaller than the critical volume fraction (Φc) 10 % w/w. However when the water content was raised above Φc = 10 % w/w, the value of conductivity increases fast up to Φw = 50 % w/w. This phenomenon is known as percolation and the critical Φw at which it occurs is known as percolation threshold (Φp = 10 % w/w). At Φw > 50 % w/w, it increases continuously, but with relatively moderate rate up to a maximum Φw = 60 % w/w. This shows that the conductivity of the system was not significantly affected by further addition of water. After reaching the maximum value, the conductivity of the system was slightly decreased by further addition of the water phase. This suggested the further dilution of o/w ME with the added water which decreased the concentration of the dispersed oil droplets. Thus, the σ–Φw curve illustrates the occurrence of the three structural regions: w/o (Φw < 10 % w/w), non-spherical w/o-bicontinuous-non-spherical o/w (10 % w/w < Φw < 50 % w/w) and o/w (Φw > 50 % w/w). The conductivity results obtained for plain and ITTN-loaded ME formulations (Tables 1 and 2) showed that loading ITTN and the addition of water phase into the formulations had no negative effects on system stability. When an unstable emulsion system and phase separation occurs, the conductivity values are greatly reduced (Kantarci et al. 2007). However, the conductivity values for unloaded vehicles were about two to three factors lower than those of ITTN-loaded formulations. The variation of viscosity as a function of water content along the dilution line (A–B) for the system IPM/LAB/PO/water (Fig. 1) is presented in Fig. 4b. All the tested samples were liquids of low viscosity. Three regions were observed in this curve. In the first region, where the water volume content is below 10 % w/w, the viscosity increases. Then a sharp decrease is observed at water volume fractions above 10 % w/w. For water contents between 10 and 40 % w/w, the viscosity of the ME systems decreases slowly. A slight increase in viscosity is also observed with increase in the water volume fraction from 40 to 55 % w/w. A sudden decrease in the viscosity is observed with increase in the water volume fraction above 55 % w/w. The viscosity is sensitive to interactions and it is rather an interesting technique to study interactions in systems of known structure. The change in viscosity with the increase in water volume fractions should be correlated to structural effects due to interfacial packing (Biruss and Valenta 2008; Fanun 2008; Kantarci et al. 2007). For water content below 10 % w/w, the increase in viscosity indicates increase of volume fraction of dispersed phase and reflects a transformation of system microstructure due to growth of spherical droplets into larger non-spherical droplets by an attractive interaction as a function of Φw. The position of the maximum on the η−Φw curve is at water phase of about 10 % w/w. The reduction in the viscosity with the increase in water content above 10 % w/w to about 60 % w/w seems to be attributed to the fact that the emulsifiers move from the bulk to the interface to cover the water and oil in a bicontinuous structure. The small increase in the dynamic viscosity for water contents above 40 % w/w suggests a structural transformation from a bicontinuous structure to oil in water structure. This phenomenon can also be explained in terms of a decrease in the hydrophobic interaction of the emulsifier tails. High water contents cause a reduction in the inter-droplet interactions and reduction in the viscosity. The relatively low viscosity values indicate that the MEs formulated are composed of individual spherical droplets or bicontinuous structures and no anisometric aggregates are present (Patel et al. 2011b). The results of viscosity measurements for plain and ITTN-loaded ME formulations (Tables 1 and 2) indicate that the viscosity values of the ITTN-loaded MEs were higher than the unloaded vehicles. The conductivity and viscosity data confirmed the continuous structural transitions during the increase of water phase volume fraction in the selected oil/emulsifier/co-emulsifier mixture.
In vitro skin permeation studies were conducted to measure the rate and extent of compound transport across the skin. Figure 5(A) shows the release profiles of ITTN from the ME formulations. The cumulative amount of ITTN that had permeated through rat skin (μg/cm2) was plotted as a function of time (hours). It is possible to calculate the steady-state flux (Jss) obtained from the slope of the linear portion (2–8 h) of the graph (Bachhav and Patravale 2009; Djordjevic 2004; Patel et al. 2009, 2011b; Shah et al. 2007). The permeation parameters of the tested ME formulations are presented in Table 3.
A steady increase of ITTN in the receptor chamber with time was observed. The permeation profiles of MEs followed zero-order kinetics. The depletion of ITTN in the external phase because of the permeation into the skin can be supplemented by the release of the ITTN from the internal phase. Thus, the zero-order release kinetics and sustained and controlled delivery of ITTN were obtained (Bachhav and Patravale 2009; Djordjevic 2004; Patel et al. 2009, 2011b, 2013a).
When all the formulations containing IPM as an oil phase were evaluated according to the release rate of ITTN, I6 showed the highest flux value (0.0380 ± 0.0057 μg/cm2/h) and I1 showed the lowest flux value (0.0318 ± 0.0021 μg/cm2/h). However, there was no significant difference between flux values of all the formulations. From Table 3, it can be concluded that the permeation rate of ITTN increases with decrease in the concentration of oil and Emix and increase in the concentration of water (Bachhav and Patravale 2009; Djordjevic 2004; Patel et al. 2009, 2011b, 2013a).
Due to low viscosity of MEs, the mobility of drugs in MEs is more facile and this is important for the permeation into skin. So the water content of ME formulations was varied between 10 and 60 %, as there exists a really tight relationship between the hydration effect on the stratum corneum and dermal permeation (Biruss and Valenta 2008; Fanun 2008; Kantarci et al. 2007).
It was concluded that the content of Emix in ME affected the skin permeation rate of ITTN significantly. As the content of Emix was decreased from 72 to 32 % w/w, the skin permeation rate of ITTN was also increased. This may be due to increased thermodynamic activity of the drug in the ME at the lower content of Emix, as ITTN is soluble in Emix. To evaluate the effect of oil phase on the permeation rates, different MEs containing IPM concentration from 8–18 % w/w were compared. The ME I6 (containing 8 % w/w oil phase) showed the highest permeation rates. The increase in oil phase in the vehicles resulted in decrease of permeation rate. It is considered that a high content of Emix in the MEs (I1, I2, I3, I4, and I5) may make the effect of oil on the skin permeation less pronounced. Also, the high solubility of drug in the oil phase could lead to an unfavorable partition from the oil phase (Patel et al. 2009, 2011b, 2013a, b, c). Thus, it was concluded that the high permeation of MEs may be attributed to the combined effects of both the lipophilic and hydrophilic domains of MEs. Large microparticles (>10–20 μm) do not penetrate the skin and remain on the stratum corneum, but particles of <3 μm are randomly distributed in the stratum corneum and penetrate the skin (Shah et al. 2007) When the particle size is very small, the number of vesicles that can interact on a fixed area of stratum corneum may be increased, thereby increasing its efficiency in the percutaneous uptake of drugs. The lower permeation of ITTN from ME containing larger size particles as compared to ME with smaller particles may be explained by the above-mentioned facts (Fanun 2008).
ME showed good shelf stability and no phase separation, cloudiness or any flocculation or precipitation for 6 months. These samples also showed no sign of phase separation under stress when subjected to centrifugation at 5000×g for 30 min. Thus centrifuge tests showed that all MEs had good physical stability. All physicochemical parameters were determined at weekly intervals for the first month and monthly interval for the subsequent months. However, no significant change in the physicochemical parameters was observed (data not shown).
Development and evaluation of microemulsion-based gel
The formulation I6 (containing 8 % w/w IPM, 32 % w/w Emix, 60 % w/w water) having appropriate physicochemical parameters and higher permeation parameters was considered as the optimized formulation. The MBG of the optimized formulation was developed using a suitable polymer capable of modifying the rheological behaviour. Various gelling agents, namely hydroxypropyl methylcellulose (Methocel K4 M) and non-benzene grade Carbopol® polymers (Carbopol® 971P NF, Carbopol® 974P NF and Carbopol® 980P NF) were evaluated for their ability to gel optimized ITTN-ME. Also, different concentrations of the above-mentioned gelling agents were tried. The suitable gelling agent was selected on the basis of compatibility with ME structure, feel and ease of spreadability. When hydroxypropyl methylcellulose (Methocel K4 M) and Carbopol® 974P NF were added to ME to prepare MBG, only an ivory-white gel was obtained indicating that the structure was disturbed. So it was concluded that they were not a suitable gel matrix for this ME system. The incorporation of Carbopol® 980P NF and Carbopol® 971P NF into ME could increase the viscosity and also maintain the ME structure. Further, since MBG containing Carbopol® 971P NF had the most appropriate fluidity and spreadability for topical administration, it was selected as an optimum gel matrix for optimized ME (Bachhav and Patravale 2009).
Briefly, the MBG of I6 was prepared as shown in Table 4. The influence of the method of addition of Carbopol® 971P NF on the formation of MBG was investigated. Carbopol® 971P NF was directly added into ME or the aqueous phase of ME, respectively (Bachhav and Patravale 2009).
As per the above observations, it can be concluded that the gel network increased the viscosity of the system, with no influence on the spontaneous dispersion of oily phase in aqueous phase or the spontaneous formation of ME. The order of addition of Carbopol® 971P NF had no significant influence on the formation of MBG, but might influence the homogenized swelling of Carbopol® 971P NF. In a subsequent study, MBG was prepared by mixing the swollen gel matrix with the oily phase.
Further, the influence of the different concentrations of Carbopol® 971P NF on the viscosity of ME was evaluated. 0.5, 1.0, 1.5 and 2 % w/w Carbopol® 971P NF was swollen in the aqueous phase. After TEA was used to adjust pH of the swollen gel matrices, MBG was prepared by mixing the oily phase with various gel matrices. The incorporation of Carbopol® 971P NF into ME resulted in a significant increase of viscosity. The viscosity of MBG containing 0.5, 1.0, 1.5 and 2 % w/w Carbopol® 971P NF was 0.7 × 103 cp, 1.8 × 103 cp, 3.1 × 103 cp and 6.5 × 103 cp, respectively. MBG containing 0.5 % Carbopol® 971P NF had a relatively high fluidity. However, 2 % w/w Carbopol® 971P NF resulted in the most appropriate fluidity for topical administration. So, 2 % w/w Carbopol® 971P NF as the optimum gel matrix was added to ME I6 to obtain MBG containing ITTN.
The ITTN content of MBG was found to be 98.97 ± 0.053 % w/w of the theoretical value (0.05 % w/w). The pH of the gel was 6.2 ± 0.03 which is in the physiological range. Rheology is less precise, but a simpler way to identify anisotropic aggregates in the system. In ME, the formation of liquid crystalline stage coincides with the formation of nonspherical aggregates (cylindrical or lamellar aggregates), which obstructs the flow in the dispersion medium. This produces high yield value (Biruss and Valenta 2008; Djekic and Primorac 2008; Fanun 2008; Kantarci et al. 2007). MEs being isotropic (spherical) systems offer less resistance to flow and exhibit low viscosity as compared to macroemulsions. The rheological properties (study of deformation and flow of matter) are vital in various pharmaceutical areas. It helps to monitor the effect of vehicles consistency on release of drug from the preparations and subsequent percutaneous absorption. It is also important from the manufacturing point of view. For a pharmaceutical or cosmetic product to be spread easily on the skin without running, it must be neither too fluid nor too viscous and show plastic or pseudoplastic rheological behavior (Bachhav and Patravale 2009; Kantarci et al. 2007). The ITTN-MBG showed pseudoplastic behavior which facilitates the flow and improves the spreading characteristics of the formulation, and the viscosity of MBG at 5 rpm was found to be 6.5 × 103 ± 0.2 × 103 cp.
Gel spreadability is an important parameter. Application of the formulation on the inflamed part would be more comfortable if the base spreads easily, exhibiting maximum slip and drag. When a weighed quantity of gel was placed in between two glass plates of known weight, it spread uniformly to produce a circle, the diameter of which was related to its spreadability; the larger the diameter, the better was the spreadability (Bachhav and Patravale 2009; Kantarci et al. 2007). The diameter of MBG was found to be 7.2 ± 0.01 cm, while the diameter of the marketed gel was found to be 5.8 ± 0.02 cm indicating that the spreadability of MBG was better than that of conventional gel. This might be because of the loose gel matrix nature of MBG formulation due to the presence of oil globules rather than the conventional gel matrix.
MBG was found to be stable at room temperature for 6 months with no significant change in the viscosity (6.7 × 103 ± 0.8 × 103 cp), spreadability (7.0 ± 0.01 cm), pH (6.4 ± 0.05) and drug content (98.87 ± 0.043 % w/w). The centrifuge tests indicated that it had good physical stability.
To assess the skin retention and penetration of ITTN from MBG, the in vitro permeation ability through skins and into skins were assessed using Franz diffusion cells. The in vitro permeation of ITTN through rat skin from MBG and marketed gel (Sotret®) was calculated in terms of mean cumulative amount diffused at each sampling time point during a time period of 12 h (Fig. 5b). The permeation parameters of MBG and marketed gel are presented in Table 5. To understand the mechanism of drug release from these formulations, the data were treated according to zero-order (cumulative amount of drug released vs time), first order (log of the cumulative amount of drug released v/s time) and higuchi (cumulative amount of drug released v/s square root of time) equation. Moreover the plot of cumulative amount of drug released vs time showed a linear relationship (r 2 = 0.9875) in case of ITTN-MBG, thus indicating that ITTN permeation followed zero-order kinetics (Table 5). In the case of marketed gel the plot of cumulative amount of drug released vs square root of time showed a linear relationship (r 2 = 0.9806), indicating that ITTN permeation followed Higuchi model (Table 5).
Although the authors did not attempt to establish the mechanism of ITTN permeation through skin from MBG, we believe that the static droplets could come in close contact with the skin due to adhesiveness of the polymer and a large amount of inner IPM might penetrate into the stratum corneum due to the small diameter of droplets. This phenomenon has been hypothesized in some investigations (Biruss and Valenta 2008; Kantarci et al. 2007; Escribano et al. 2005). Though the flux value of ITTN-based gel (0.0265 ± 0.0017 μg/cm2/h) is lesser than marketed ITTN gel (0.0284 ± 0.0047 μg/cm2/h), the difference is not significant (P > 0.05). Thus it was hypothesized that MBG may avoid the systemic uptake of ITTN in comparison to marketed gel and might present a potential to avoid systemic adverse side effect.
Also, the flux value of MBG of I6 (0.0265 ± 0.0017 μg/cm2/h) was found to be less than ME of I6 (0.0380 ± 0.0057 μg/cm2/h) (P < 0.05). In literature, it has been reported that the release of drug from viscosized ME was higher than that from fluid ME. But our results were not in accordance with previously published studies (31, 32). This might be attributed to gel formation in ME, by the addition of Carbopol® 971P NF that will increase its viscosity, transform the microstructure of ME to lamellar or a highly ordered microstructure and further decrease the permeation in the skin (Huang et al. 2008).
Infrared study
The infrared spectra of ITTN pure powder, plain ME, IME and MBG are as shown in the Fig. 6a–d. ITTN was characterized by bands around 1700–1500 and 1300–1100 cm−1 that correspond to C = O and C–O stretching vibrations. The IR spectrum of ITTN-ME and ITTN-MBG is entirely different from ITTN, while it closely resembles the spectrum of unloaded ME. The characteristic bands of ITTN either disappeared or few bands of ITTN were present with reduced intensity probably due to the restriction inside the formulation matrix. The absorption band at 3400 cm−1 due to hydroxyl stretching was not affected in ITTN-ME and ITTN-MBG, which emphasized the absence of any possible interaction between the drug and the formulation components used (Patel et al. 2013c).
Skin retention study
The accumulative amounts of ITTN in skin from MBG and marketed gel were 3.53 ± 0.07 μg and 3.02 ± 0.04 μg, respectively. MBG could significantly (P < 0.05) increase the accumulative uptake of ITTN in skin compared to the marketed formulation. This result supported our hypothesis made in skin permeation studies on rat skin. The more ITTN permeates, the less is it retained in the skin and might lead to systemic adverse side effects (Liu et al. 2007). ME has been shown to improve the dermal localization of several topical therapeutic agents. This was one of the reasons for employing the ME approach for topical delivery of ITTN, as its epidermal localization is highly desirable for enhancing the treatment of skin diseases such as psoriasis, acne, photoaging and epithelial skin cancer (Liu et al. 2007).
Histopathological investigation
The histology of excised rat skin in the control and that treated with optimized ME, MBG and marketed gel is shown in Fig. 7a–d. The microscopic observations indicate that the optimized ME (ITTN-ME), MBG (ITTN-MBG) and marketed gel had no significant effect on the microscopic structure of the skin. The surface epithelium lining and the granular cellular structure of the skin were totally intact. No major changes in the ultrastructure of skin morphology could be seen and the epithelial cells appeared mostly unchanged (Patel et al. 2009, 2011b, 2013a, b, c).
Skin irritation testing
Skin irritation limits the utility and acceptability of ITTN therapy by patients. Most of the currently marketed conventional dosage forms are not able to reduce the irritation caused by topical application of ITTN. Hence, we aimed to formulate a delivery system of ITTN which was able to reduce erythema (skin irritation). It was hypothesized that encapsulation of ITTN in ME would reduce the contact of the acidic function (–COOH) of ITTN (the triggering factor for erythematic events) with the stratum corneum, thus resulting in reduced erythematic episodes (Draize et al. 1944). The results obtained from the primary skin irritation studies are listed in Table 6 and the actual photographs are depicted in Fig. 8a–d. Draize patch test is a reliable method and the results obtained from this study can be linked to those obtained in humans (Biruss and Valenta 2008; Kantarci et al. 2007; Escribano et al. 2005). The skin irritation studies indicated that MBG containing ITTN resulted in less irritation as compared to marketed ITTN formulation (Sotret® gel) after 72 h of application (Table 6). Thus, MBG demonstrated advantage over marketed formulation in improving the skin tolerability of ITTN, indicating their potential in improving patient acceptance and topical delivery of TTN.
Conclusion
Collectively, the results demonstrate that MBG may be a more promising approach for the topical delivery of ITTN. It is assumed that MBG due to its appropriate physicochemical properties, high skin permeation ability, higher accumulative uptake in skin and improved skin tolerability may be more suitable for targeted therapy, which could be developed as a novel regime for topical administration of ITTN. Nevertheless, significant work is yet to be conducted to elucidate the possible mechanism(s) of drug delivery into the skin. Clinical investigations with emphasis on the desired therapeutic efficacy and favorable safety profile followed by pilot-scale studies on manufacturing the product for commercial use also need to be considered.
References
Alvarez-Figueroa MJ, Blanco-M´endez J (2001) Transdermal delivery of methotrexate: iontophoretic delivery from hydrogels and passive delivery from microemulsions. Int J Pharm 215:57–65
Bachhav YG, Patravale VB (2009) Microemulsion based vaginal gel of fluconazole: formulation, in vitro and in vivo evaluation. Int J Pharm 365:175–179
Biruss B, Valenta C (2008) The advantage of polymer addition to a non-ionic oil in water microemulsion for the dermal delivery of progesterone. Int J Pharm 349:269–273
Chen H, Mou D, Du D, Chang X, Zhu D, Liu H, Xu H, Yang X (2007) Hydrogel-thickened microemulsion for topical administration of drug molecule at an extremely low concentration. Int J Pharm 341:78–84
Djekic L, Primorac M (2008) The influence of cosurfactants and oils on the formation of pharmaceutical microemulsions based on PEG-8 caprylic/capric glycerides. Int J Pharm 352:231–239
Djordjevic L, Primorac M, Stupar M, Krasjinik D (2004) Characterization of caprylocaproyl macrogolglycerides based microemulsion drug delivery vehicles for an amphiphilic drug. Int J Pharm 271:11–19
Draize J, Woodard G, Calvery H (1944) Methods for the study of irritation and toxicity of substances topically applied to skin and mucous membranes. J Pharmacol Exp Ther 82:377–390
Escribano E, Obach M, Arevalo MI, Domenech J, Queralt J (2005) Rapid human skin permeation and topical anaesthetic activity of a new amethocaine microemulsion. Skin Pharmacol Physiol. 18:294–300
Fanun M (2008) Phase behavior, transport, diffusion and structural parameters of nonionic microemulsions. J Mol Liquids. 139:14–22
Huang YB, Lin YH, Lu TM, Wang RJ, Tsai YH, Wu PC (2008) Transdermal delivery of capsaicin derivative-sodium nonivamide acetate using microemulsions as vehicles. Int J Pharm 349:206–211
Kantarci G, Ozguney IS, Karasulu HY, Arzik S, Guneri T (2007) Comparison of different water/oil microemulsions containing diclofenac sodium: preparation, characterization, release rate and skin irritation studies. AAPS Pharm Sci Tech. 8:75–81
Liu J, Hub W, Chen H, Ni Q, Xu H, Yang X (2007) Isotretinoin-loaded solid lipid nanoparticles with skin targeting for topical delivery. Int J Pharm 328:191–195
Patel MR, Patel RB, Parikh JR, Solanki AB, Patel BG (2009) Effect of formulation components on the in vitro permeation of microemulsion drug delivery system of fluconazole. AAPS Pharm Sci Tech 10:917–923
Patel MR, Patel RB, Parikh JR, Patel BG (2011a) HPTLC method for estimation of isotretinoin in topical formulations, equilibrium solubility screening and In Vitro permeation study. J Liq Chromatogr Rel Tech 34:1783–1799
Patel MR, Patel RB, Parikh JR, Solanki AB, Patel BG (2011b) Investigating effect of microemulsion components: in vitro permeation of ketoconazole. Pharm Dev Tech 16:250–258
Patel RB, Patel MR, Bhatt KK, Patel BG (2013a) Formulation consideration and characterization of microemulsion drug delivery system for transnasal administration of Carbamazepine. Bul Fac Pharm Cairo Uni 51:243–253
Patel RB, Patel MR, Bhatt KK, Patel BG (2013b) Paliperidone loaded mucoadhesive microemulsion in treatment of schizophrenia: formulation consideration. J Pharma Inn 8:195–204
Patel RB, Patel MR, Bhatt KK, Patel BG (2013c) Risperidone loaded mucoadhesive microemulsion for intranasal delivery: formulation, development, physicochemical characterization and ex vivo evaluation. J Drug Del Sci Tech 23:261–267
Russell JJ (2000) Topical therapy for acne. Am Fam Phys 61:1–13
Shah KA, Date AA, Joshi MD, Patravale VB (2007) Solid lipid nanoparticles (SLN) of tretinoin: potential in topical delivery. Int J Pharm 345:163–171
Subramanian N, Ghosal SK, Acharya A, Moulik SP (2005) Formulation and physicochemical characterization of microemulsion system using isopropyl myristate, medium chain glyceride, plysorbate 80 and water. Chem Pharm Bull 12:1530–1535
Zaenglein AL (2008) Topical retinoids in the treatment of acne vulgaris. Semin Cutan Med Surg 27:177–182
Acknowledgments
The authors are thankful to Astron Research Ltd. (Ahmedabad, India) for the gift sample of ITTN pure powder, Sophisticated Instrumentation Center for Applied Research and Testing (SICART) (Vallabh Vidyanagar, India) for providing facilities for carrying out analytical work, Gattefosse (Saint-Priest, France), Colorcon (Asia) Pvt. Ltd. (Mumbai, India), Abitec Corporation (Janesville, USA), BASF (Mumbai, India), Sasol (Witten, Germany), Lubrizol Advance Material India Pvt. Ltd. (Mumbai, India) and Noveon (Cleveland, USA) for providing gratis samples of excipients, and Dr. A. M. Thakar, Dr. Zala, Dr. Kalyani and Dr. Mathakiya, Veterinary Science College, Anand Agriculture University, Anand, for providing guidance and research assistance for skin irritation study.
Conflict of interest
The author(s) confirm that this article has no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Patel, M.R., Patel, R.B., Parikh, J.R. et al. Novel isotretinoin microemulsion-based gel for targeted topical therapy of acne: formulation consideration, skin retention and skin irritation studies. Appl Nanosci 6, 539–553 (2016). https://doi.org/10.1007/s13204-015-0457-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13204-015-0457-z