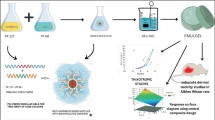
Abstract
Background
The objective of this current research was to enhance the topical delivery of Nadifloxacin (NDFX) by incorporating it into a transethosomal gel formulation. NDFX has limited penetration into the deep layer of the skin because it is poorly water soluble and it has a log p value of 2.47. To optimize the formulation, the “Box–Behnken design” was utilized. The independent variables included phosphatidylcholine 90, tween 80 and ethanol. The produced formulations underwent evaluation for entrapment efficiency, vesicle size and zeta potential. The optimized formulation was then incorporated into suitable gel bases and subjected to further investigation, including in vitro diffusion, ex vivo penetration, in vitro antimicrobial assay and in vivo anti-acne activity.
Results
The optimized formulation exhibited an entrapment efficiency of 80.12%, a vesicle size of 156.1 nm and a zeta potential of − 33.23 mV. TEM images confirmed the presence of encapsulated vesicles with a spherical shape. The in vitro diffusion study demonstrated that the transethosomal gel containing Carbopol 934 (1%) exhibited higher drug release compared to the HPMC K4M gels. Furthermore, the ex vivo permeation study revealed that the optimized transethosomal gel demonstrated increased permeation compared to the commercially available formulation.
Conclusion
The optimized transethosomal formulation displayed enhanced in vitro antimicrobial and in vivo anti-acne effects against Propionibacterium acnes in Wistar albino rats when compared to the marketed formulation.
Similar content being viewed by others
Background
Skin disorders are one of the most prevalent illnesses; skin problems afflict thousands of individuals every day and can affect anyone at any age. It was primarily brought on by various infectious bacteria or inflammatory diseases. Skin conditions include a wide range of symptoms and severity. Some may have environmental causes, while others may have genetic or dependent reasons [1]. Several infectious diseases affect the skin and hair follicles and are brought on by bacterial, fungal or viral infections. These illnesses result in skin eruption [2].
Acne vulgaris is a persistent skin disorder that affects the pilosebaceous glands of hair follicles. Comedones, inflamed papules and pustules, nodules & cysts and scaring are the different types of acne lesions. Acne vulgaris is characterized by skin with comedone and seborrhea, which is caused due to several factors: hyperkeratosis, heightened sebum production induced by androgens, inflammation and bacterial colonization of hair follicles on the face, neck, chest and back by Propionibacterium acnes. A "whitehead" is a closed comedone and a "blackhead" is an open comedone; the black color is caused due to melanin. The comedo is a non-inflamed lesion that typically recurs on its own with minimal or discolored scarring [3].
Nadifloxacin (NDFX), 7-fluoro-8-(4-hydroxy-piperidin-1-yl)-12-methyl-4-oxo-1-azatricyclo- trideca-2,5,7,9 (13)-tetraene-3-carboxylic acid (Fig. 1), is a fluorinated quinolone antibiotic and is commonly used as a topical medication for the treatment of numerous inflammatory acne lesions. It is highly effective against P. acness and other gram-negative and gram-positive bacteria. In addition to its antibacterial effects, NDFX has been reported to have anti-inflammatory effects, which may help with some elements of inflammatory acne [4].
For the treatment of acne, 1% w/w Nadifloxacin topical cream is available in the market. Unfortunately, NDFX, characterized by its low solubility in water, is a fluoroquinolone compound that possesses a log P value of 2.47 and due to the large droplet size of topical creams, NDFX has shown limited penetration into the deep layer of the skin [5], resulting in poor patient compliance and adherence to NDFX topical regimen. Several methods, including nanocarrier-based formulations and Novel lipid vesicles, referred to as ultra-deformable vesicles (UDV), like ethosomes, transferosomes and transethosomes, have already been researched to enhance the targeted administration of cosmeceuticals and pharmaceuticals [6, 7].
Transferosomes are vesicular carriers characterized by their elastic properties and lipid bilayer structure. These carriers incorporate an edge activator, which is a biocompatible surfactant. However, a significant limitation of this formulation is the challenge of effectively loading hydrophobic drugs into these vesicles without compromising their elasticity [8]. On the other hand, ethosomes are vesicular carriers composed of hydro-alcoholic phospholipids with a high concentration of alcohol. While ethosomes offer certain advantages, such as simple, passive and non-invasive drug delivery with enhanced permeation through the skin, and targeting to deeper skin layers for various skin diseases, they also have a significant disadvantage. When ethosomes applied to the skin in a non-occlusive state, it tends to cause skin dehydration due to the evaporation of ethanol from the formulation [9].
Transethosomes are ultra-deformable vesicles that are a combination of transferosomes and ethosomes and will overcome the disadvantage of both. Transethosomes, alternatively referred to as ultra-deformable liposomes, possess an irregular spherical structure and exhibit remarkable elasticity. They possess the capability to effectively encapsulate drugs with varying molecular weights, encompassing both low and high molecular weight compounds. Transethosomes structure is composed of a phospholipid, edge activator and ethanol. Phospholipid functions as a carrier for drug molecules to pass through the skin. The edge activator, a biocompatible polymer, serves as the agent responsible for softening the vesicles. It imparts flexibility to the vesicles and acts as the permeation enhancer. Ethanol gives a unique identity to the transethosomal vesicles; it deforms the epidermis layer and allows these nanocarriers to penetrate deeply into the stratum corneum through tiny openings caused by fluidization. The utilization of ethanol along with an edge activator results in the reorganization of the lipid bilayer, enhancing its flexibility and enabling it to effectively permeate the deeper layers of the dermis [10].
Hence, in the present study, a transethosomal-based gel was chosen as the carrier for the effective transdermal delivery of NDFX. However, as per the literature review, no DOE-based transethosomal formulation with improved penetration and effectiveness has been reported for NDFX. Therefore, the primary objective of the present study was to create and enhance transethosomes loaded with NDFX, employing the Box–Behnken experimental design. The formulations underwent characterization to determine their vesicle size, zeta potential, entrapment efficiency and in vitro diffusion properties. The optimized formulation was then assessed for ex vivo permeation, in vitro antimicrobial assay and in vivo anti-acne effects using an animal model induced with P. acnes.
Materials and methods
Materials
NDFX was acquired as a complimentary sample from Wockhardt Research Centre, located in Aurangabad, India. Soya Phosphatidylcholine-90 was acquired as a complimentary sample from Lipidome Lifesciences, based in Ahmedabad, India. Tween 80 was procured from Hi-Media Laboratories, Pvt. Ltd. (Mumbai, India). Ethanol was procured from S.D. Fine Chem Ltd (Mumbai, India). Carbopol 934 was obtained from LOBA Chemie Pvt. Ltd, located in Mumbai, India. Methanol and chloroform were acquired from Fisher Scientific, India. All remaining chemicals utilized were of analytical grade.
Experimental animal
Wistar rats either male or female weighing from 150 to 200 gm were selected for the study. Before commencing the study, necessary authorization and endorsement were obtained from the Institutional Animal Ethics Committee (IAEC) and the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), under the Reg. No. 221/Po/Re/S/2000/CPCSEA, during their meeting on 03/12/2022.
Methods
Preparation of NDFX-loaded transethosomes
The cold method was employed for developing NDFX-loaded transethosomes. This method involves mixing of two phases at low temperature (30 °C) in a water bath. In this method aqueous phase (edge activator + water) is added to organic phase (ethanol + phospholipid) with continuous stirring in order to form the vesicles. This method is most widely used for thermo-labile drugs, and it is easily scalable [10].
Soya phosphatidylcholine 90 (phospholipid), Tween 80 (surfactant) and Nadifloxacin (1% w/w) were dissolved in ethanol at a temperature of 30 °C, forming the organic phase. Simultaneously, the aqueous phase (consisting of Millipore water) was heated to 30 °C. The aqueous phase was then added drop by drop to the organic phase, while the mixture was continuously stirred at a speed of 1200 revolutions per minute using a magnetic stirrer (RCT Basic IKA, India). The stirring was continued for 45 min to get the transethosomal dispersions. Thereafter, transethosomal dispersion was ultrasonicated using a probe sonicator (SONICS® VCX 750, USA). The formulation was then refrigerated at 4 °C until further characterization [11].
Optimization of NDFX-loaded transethosomes by Box–Behnken design (BBD)
Based on the literature review, several parameters have been identified that may influence the ideal characteristics of transethosomes for topical delivery and it was found that three variables (factors) such as phospholipid, tween 80 and ethanol concentration might have a direct impact on the critical transethosome characteristics required for successful topical administration. The response surface methodology utilized the Design Expert software to construct a Box–Behnken design consisting of three factors with three levels each (33). This experimental design, generated with the software, comprised a total of 17 runs, including 5 repeated center points, and the resulting outcomes were subsequently measured. By employing the Box–Behnken design, the study aimed to examine the impact of phospholipid, surfactant and ethanol concentrations on dependent variables, namely entrapment efficiency, vesicle size and zeta potential. Table 1 shows the independent and dependent elements chosen for the experimental design. The NDFX-loaded transethosome vesicles were tested for vesicle size, entrapping efficiency and zeta potential [12].
Evaluation of NDFX-loaded transethosomes
Particle size, polydispersity index and zeta potential
The Malvern zeta sizer (Malvern Instruments, Worcestershire, UK) was utilized to ascertain the particle size, polydispersity index and zeta potential of the transethosomes. The measurements were taken at a constant angle of 90° and a temperature of 25 ± 2 °C. To facilitate the analysis, the samples were diluted in millipore water and the measurements were taken in triplicate. These parameters provide information regarding the size, homogeneity and stability of the vesicles [13].
Entrapment efficiency
The ultra-centrifugation technique was used for estimating the entrapment efficiency of transethosomal formulations. Two milliliters of each formulation was transferred into a microcentrifuge tube and was subjected to ultracentrifugation at 4 °C by an Ultracentrifuge (Kubota, Japan). Following that, the supernatant was extracted with a micropipette and diluted with methanol to rupture the vesicles. A UV spectrophotometer (Shimadzu -1900, Japan) was used to measure the quantity of drug in the supernatant at 295.0 nm [14].
The following formula was used to compute the % EE.
Surface morphology of optimized transethosomes
Transmission electron microscopy (TEM) was employed to examine the morphology of the prepared transethosomes. To summarize the procedure, a small amount of the diluted transethosomes sample was deposited onto a copper grid and left to air-dry. Once dried, the sample was treated with a 1% w/v solution of phosphotungstic acid for fixation and subsequently subjected to TEM analysis, with accompanying photographs captured [15].
Stability studies of optimized formulation
To determine the stability of the optimized transethosomes, short-term stability studies were conducted in compliance with ICH GCP guidelines. The prepared formulation was stored in glass vials within a humidity-controlled oven maintained at a temperature of 25 ± 2 °C and a relative humidity of 65 ± 5%. Additionally, it was refrigerated at 4 ± 2 °C with a relative humidity of 65 ± 5%. At regular intervals of 0, 15, 30 and 90 days, a sample was extracted for analysis [11].
Preparation of transethosomal gels
The preliminary studies were carried out, the gel was formulated through the dissolution of Carbopol 934 in purified water while stirring continuously; and the pH was adjusted to 6–6.5 by incorporating a 10% solution of triethanolamine. HPMC K4M was dispersed in purified water and left overnight. To create the transethosomal gel, the previously prepared transethosomal dispersion was incorporated into the gel in a 1:1 ratio with adequate stirring. The formulation details for gel bases are provided in Table 2 [16, 17].
Evaluation of transethosomal gels
Determination of pH
In order to ascertain the pH of multiple gels, a digital pH meter was employed. Firstly, 500 mg of pre-prepared transethosomal gels was dissolved in 20 milliliters of distilled water. The resulting mixture was stirred for a duration of 30 min at room temperature using a magnetic stirrer. Subsequently, the pH sensor probe electrode was immersed in the dissolved gel and the pH value of the formulation was recorded from the digital screen [17].
Determination of viscosity
The viscosity of the optimized transethosomal gel formulation was estimated using a Brookfield viscometer. After applying the transethosomal gel formulation, it was allowed to settle for 5 min. After that, spindle number one was revolved at 50 revolutions per minute at a temperature of 25 ± 2 °C [18].
Determination of spreadability
The spreadability of the transethosomal gel was assessed using the glass slide technique. A precisely measured 1.0 g of gel was positioned at the center of a glass slide measuring 10 × 5 cm. Another slide of the same dimensions was placed on top of it. To ensure uniform compression and maintain a consistent thickness, a weight of 100 g was applied to the upper slide for a duration of 5 min. The time required for the glass slide to move 6 cm and separate from the lower glass slide was recorded [19, 20]. The spreadability of the gel was then calculated using the following formula:
where ‘S’ represents Spreadability, ‘M’ represents the weight applied to the upper slide, ‘L’ represents the distance traveled by the slide (6 cm), and ‘T’ represents the time taken in seconds.
Determination of drug content
In a 50-ml volumetric flask, 1 gm of gel was dissolved with 50 ml methanol. The solution underwent sonication in a bath until a transparent solution was achieved. Subsequently, the solution was filtered through a 0.45-µm filter and appropriately diluted using methanol. The drug concentration was determined by measuring absorbance with a UV spectrophotometer at the wavelength of 296 nm, using methanol as the reference solution [19].
In vitro drug diffusion study
The Franz diffusion cell was utilized to determine the in vitro diffusion of drugs from different transethosomal gel formulations. To activate the dialysis membrane, it was previously soaked in pH 7.4 buffer. The receptor chamber was then filled with 12 ml of phosphate buffer at pH 7.4, which served as the medium for diffusion in the receptor compartment. The gel formulation, equivalent to 2 mg of the drug, was accurately weighed and thronged in the preactivated dialysis membrane. Throughout the experiment, the receptor medium was maintained at a temperature of 37 ± 2 °C and stirring was consistently performed at 100 rpm. At specific time intervals of 0.5, 1, 2, 4 and 8 h, 0.5 ml samples were withdrawn from the cell and replaced with fresh medium. The quantification of drug release was carried out using a UV spectrophotometer at a wavelength of 291 nm [21].
Ex vivo permeation study
A preliminary study was conducted to examine skin permeation using rat skin and the Franz diffusion cell, which had an effective permeation area of 1.76 cm2. Prior to the permeation study, the rat skin underwent a preparation process. Initially, the hair on the skin was eliminated using an electronic trimmer, followed by the removal of subcutaneous tissue through surgical means. Isopropyl alcohol was used to remove the fat from the dermis side of the skin. Afterward, the skin was washed with PBS and stored at − 20 °C in a deep freezer until it was ready for use. During the experiment, rat skin was affixed onto a diffusion cell with the dermis side facing the receiver compartment and the stratum corneum side facing the donor compartment. The receiver compartment contained a medium of PBS with a pH of 7.4. The temperature in the receiver compartment was maintained at 37 ± 0.5 °C, and throughout the experiment, it was stirred using a magnetic bead at a speed of 100 rpm. At predetermined time intervals of 0.5, 1, 2, 4 and 8 h, 0.5 ml samples were withdrawn and replaced with fresh medium. The collected samples were then analyzed for drug content using a UV spectrophotometer [22].
In vitro anti-bacterial study
Zone of inhibition The cup plate technique was used to investigate the zone of inhibition of prepared NDFX-loaded transethosomal gel against Propionibacterium acne. The Mueller–Hinton agar plates were prepared and sterilized at 121 °C and at a pressure of 15 lb. for 20 min. Subsequently, 25 ml of Mueller–Hinton agar was carefully poured into each Petri dish, allowing it to solidify. Following that, the Petri dishes were streaked with a suspension of Propionibacterium acne. Using a cork borer, four holes, each measuring 6 mm in diameter, were created on every plate. In each of these holes, the following substances were placed: a transethosomal formulation loaded with NDFX at a concentration of 10 µg (test), a commercially available NDFX cream at a concentration of 10 µg (standard), 100 µl of a blank transethosomal formulation (blank) and a negative control consisting solely of the vehicle (ethanol and buffer). The plates were incubated at 37 °C for 48 h in an anaerobic condition. The zone of inhibition was measured, and the antimicrobial activity of each formulation toward the P. acnes was investigated [23,24,25].
Animal study
Skin irritation study
A skin irritancy test was conducted on the hairless backs of Wistar rats weighing between 180 and 250 g. The purpose of the test was to determine the irritant or toxic effects of the formulated transethosomal gel on the skin. The animals were segregated into two distinct groups, with each group consisting of six test subjects, as indicated in Table 3. Group I received an application of Optimized NDFX transethosomal gel, whereas Group II received blank transethosomal gel. Prior to the application of gel, the dorsal region of the Wistar rat was trimmed using an electronic trimmer to remove the hair, followed by the application of gel onto the hairless area measuring 5 cm2. A thorough examination was conducted on the rat's skin to identify any signs of erythema and edema. After 1 h, 24 h, 48 h and 7th day of application of test substances, finally, as per the Draize protocol the scoring was given and the skin irritancy potential of the formulated transethosomal gel was determined [26,27,28].
Anti-acne study
Animal model
Wistar albino rats either male or female weighing between 150 and 200 g were employed to examine the potential anti-acne effects of a transethosomal gel loaded with NDFX. These rats were acclimated to the prescribed rehabilitation conditions for a minimum of seven days prior to the experiment.
Bacterial sample
The bacterial strain of Propionibacterium acne was cultivated within an anaerobic gas chamber using brain heart infusion (BHI) broth. The culture was incubated for 48 h at 37 °C, after which the BHI agar plate was used for subsequent culturing bacterial strain. A single colony was selected from the BHI agar plate and introduced into a PBS solution. The turbidity of the suspension was assessed using the 0.5 McFarland (1.5 × 108 CFU/ml) scale. This bacterial suspension is now prepared for injection.
Injection of bacteria
An anesthetized Wistar rat was administered a subcutaneous injection of Propionibacterium acne bacterial suspension, measuring 20 µl, into its right ear. As a vehicle control, phosphate buffer saline was injected into the left ear [29].
Treatment approach
To investigate the anti-acne potential of prepared experimental gel (NDFX-loaded transethosomal gel) in animal models, the Wistar rats were categorized into four groups, with each group consisting of six animals, as depicted in Table 4. In each group, the rats received an injection of the aforementioned bacterial suspension of P. acnes in their left ear, while their right ear was injected with phosphate buffer saline. Initially, no treatment was given to all the groups for the period of seven days (inducing period). Following a period of seven days with no treatment provided to Group I (disease control), Group II was chosen as the benchmark and subjected to treatment using the commercially available anti-acne formulation known as Nadibact cream (containing 1% w/w active ingredient); Group III and Group IV were treated with the prepared NDFX-loaded transethosomal gel and plain NDFX gel, respectively. The treatment was continued till the 14th day for groups II, III, IV, and the measurement was taken using a Vernier Caliper daily, the ear thickness was measured on a predetermined day, and the formula used to calculate the percentage change in ear thickness is as follows
where TAfter represents the thickness of the auricle subsequent to the injection, while TBefore denotes the thickness of the auricle prior to the injection [30].
Histopathological investigation
Once the anti-acne study was completed successfully on the 14th day, three animals from each group were chosen at random and killed by euthanasia. Prior to sectioning, the ear was surgically removed and immersed in a 10% formalin solution. Subsequently, the sections were treated with hematoxylin and eosin dye for staining. The stained ear slices were then placed on glass slides and examined using an optical microscope [30].
Post-treatment microbiological assay
Upon successful completion of the anti-acne study, the animals from each group were euthanized and the treated area on the right ear was gently wiped with a cotton swab soaked in ethanol before being excised. The excised ear was then divided into small pieces and homogenized in 5 ml of phosphate buffer saline using a tissue homogenizer. Subsequently, the homogenized saline solution was appropriately diluted. A small portion of the homogenate was extracted and evenly spread onto a sterilized cotton swab, which was used to inoculate a Mueller–Hinton agar plate. The plate was then incubated under anaerobic conditions at 37 °C. The number of colony-forming units (CFUs) present on the agar plate was subsequently counted in order to investigate the pharmacodynamic activity of the formulation [25].
Results
Optimization of NDFX-loaded transethosomes by Box–Behnken design (BBD)
The BBD design was used to optimize the NDFX-loaded transethosomal formulation and investigate the impact of independent factors such as soya phosphatidylcholine 90 (A), Tween 80 (B) and the ethanol (C) concentration on the dependent responses, i.e., entrapment efficiency (Y1), vesicle size (Y2), zeta potential (Y3). The experimental design (BBD) has generated 17 batches with 5 center points. The outcomes derived from these experiments are presented in Table 5. The measured values for the dependent factors, namely entrapment efficiency, vesicle size and zeta potential, ranged from 66.43 to 81.87%, 97.04–356.02 nm and − 17.12 to − 34.34 mV, respectively. The responses were subsequently subjected to statistical analysis through response surface analysis employing ANOVA. By examining the 3D surface plot and polynomial equation, the impact of independent factors on dependent responses was explored. The quadratic model proved to be the most suitable fit for analyzing entrapment efficiency and vesicle size, while the reduced quadratic model was found to be the optimal fit for examining zeta potential. The fit statistics results are given in Table 6 showing satisfactory R2, adjusted R2, predicted R2, S.D. and % C.V. [12, 19].
Effect of independent factors on response Y 1 (entrapment efficiency)
In all 17 experimental runs, the EE was found to be in the range of 43.64–81.05%.
As per polynomial Eq. (1) and 3D response plot (Fig. 2a), a discovery was made indicating that soya phosphatidylcholine 90 has a favorable impact on the entrapment efficiency of NDFX, while both tween 80 and ethanol were observed to have a detrimental effect on the entrapment efficiency. The progressive enhancement of entrapment efficiency as the concentration of soya phosphatidylcholine 90 increases can be attributed to the lipophilic nature of NDFX. This is because of the affinity of lipophilic drugs toward the lipophilic phase, resulting in their subsequent deposition therein [22].
In formulations F1 and F10, the entrapment efficiency increases from 54.71 to 67.48% as the concentration of phosphatidylcholine increases from 2 to 4%, respectively. Similar results were found when a comparison was made between formulations F4 and F17.
Entrapment efficiency decreases as the concentration of tween 80 increases. Formulation F13 (tween 80 0.2%) showed an entrapment efficiency of 72.82%, while formulation F4 (tween 80 0.4%) showed an entrapment efficiency of 57.7%. Similar results were found between the formulation F16 (tween 80 0.2%) and F17 (tween 80 0.4%). The possible cause of this phenomenon is the increased surfactant concentration, which may result in the formation of micelles alongside the vesicles in the formulation; micelles typically exhibit diminished entrapment efficiency in comparison with vesicles. Additionally tween 80 is hydrophilic in nature whose increased concentration may decrease the encapsulation of hydrophobic drug [31].
Ethanol also had an opposite influence on NDFX entrapment efficiency in transethosomes vesicles. Formulation F15 having ethanol concentration 20% showed an entrapment efficiency of 79.95%, while formulation F1 having 30% ethanol showed 54.71% entrapment efficiency. This might be due to the leaking of the transethosomal vesicles at high ethanol concentrations [32].
Effect of independent factors on response Y 2 (vesicle size)
The vesicle size was falling in the range of 97.12–187.34 nm in all 17 batches.
Based on polynomial Eq. (2) and the 3D response plot (Fig. 2b), it was observed that soya phosphatidylcholine 90 exhibited a positive impact, while Tween 80 and ethanol had a negative impact on the size of the transethosomes vesicle.
It was discovered that as the concentration of soya phosphatidylcholine 90 was increased from 2 to 4%, the vesicle size was also increased from 100.65 nm (F1) to 142.21 (F6). Similar results were seen with F13 (151.18 nm) and F16 (187.34 nm). The tween 80 was showing an inverse effect in the vesicle size of transethosomes. When the concentration of tween 80 was increased from 0.2 to 0.4%, the vesicle size of transethosomes was reduced from 187.34 nm (F16) to 160.17 (F17). The formulation F3 (173.01 nm) to F6 (142.25 nm) also presented similar results. The vesicle size of the transethosome decreases from 188.51 to 142.21 nm as the ethanol concentration increases from 20% (F12) to 30% (F10). This could be attributed to the fluidizing ability of ethanol on the phospholipid membrane of the vesicles by its interpenetrating hydrocarbon chain as the wall thickness of the vesicular membrane decreases [12].
Effect of independent factors on response Y 3 (Zeta potential)
The zeta potential in all 17 formulations was falling in the range of − 17.12 to − 34.34 mV.
The impact of soya phosphatidylcholine, tween 80 and ethanol concentration on the zeta potential of transethosomal vesicles was noted during the observation. As per the polynomial equation, the zeta potential of the vesicles (Fig. 2c) exhibited a positive impact from all three factors. The zeta potential was observed to be increasing from − 32.25(F1) to − 22.43 (F10) with increasing concentrations of phosphatidylcholine 90 from 2 to 4%. As the concentration of tween 80 was increased from 0.2 to 0.4% the zeta potential was also increased from − 27.74 (F9) to − 24.72 (F11).
The zeta potential is the critical characteristic that influences vesicular features such as the interaction of vesicles with the skin and the stability of transethosomes. According to the findings presented in Table 5, it has been observed that all the formulated transethosomal preparations exhibited a zeta potential characterized by a negative charge. Negatively charged vesicles exhibit superior skin permeability compared to positively charged vesicles [33]. The negative zeta potential observed in transethosomes primarily arises from the presence of ethanol within these nanocarriers. Ethanol induces negative charges on the polar head groups of phospholipids, leading to electrostatic repulsion. This would limit vesicle agglomeration and hence increase the stability of these transethosomal nanocarriers [11].
In the present investigation, the point prediction approach of the BBD design in the response surface methodology was used to optimize the NDFX-loaded transethosomal formulation. The formulation composition of soya phosphatidylcholine (2.03%), Tween 80 (0.3%) and ethanol (20%) met the requirements for an optimal formulation.
The optimized formulation had shown an entrapment efficiency of 79.8%, vesicle size of 150.18 nm, zeta potential of − 33.85 mV, and desirability was found to be 0.969. The results of the optimized transethosome formulation are shown in Table 7 (Fig. 3). All the observed responses of optimized transethosomal formulation close proximity to the value were predicted by Design Expert software.
Surface morphology of optimized transethosomes
The analysis using transmission electron microscopy (TEM) of the optimized transethosome formulation containing NDFX (Fig. 3c) indicated that the vesicles exhibit a spherical shape, displaying a consistent size distribution and a distinct, tightly sealed structure [21].
Stability studies
The findings from the stability study of optimized NDFX transethosomal formulation are demonstrated in Table 8. It was observed that after the completion of 90-day storage period, there were negligible alterations (p > 0.05) in the physical characteristic of transethosomes. The vesicle size was found to be increased by 5 nm, entrapment efficiency was decreased by 4%, and the zeta potential was increased by 3 mV after the completion of 90-day storage period at 25 °C ± 2 °C (room temperature condition) [34].
Characterization of NDFX-Loaded transethosomal gel
The enhanced transethosomal formulation was integrated into the secondary carrier (Gel) to make the formulation rheologically acceptable for topical administration. The gel was prepared using varying concentrations of Carbopol 934 and HPMC K4M and investigated for gel characterization studies as shown in Table 9. The pH, viscosity, spreadability and drug content were falling in the range of 6.31–7.12, 794–1112cP, 9.2–12.56 g cm2/s and 84–92%, respectively.
In vitro drug diffusion study
The investigation of the drug diffusion study of prepared transethosomal gels was carried out using the Franz diffusion cell. The drug release was found to be in the range of 42.67–64.83% after 8 h. Figure 4 illustrates the in vitro drug diffusion profile of NDFX from different transethosomal formulations. The release of NDFX from these formulations can be ranked in the following descending order: TEG1 > TEG2 > TEG3 > TEG4. As the TEG1 was found to be showing satisfactory gel characterization results and the highest drug release, it was selected as an optimized gel base for further study [16].
Ex vivo permeation study
The skin permeation study of the optimized transethosomal gel formulation TEG1, loaded with NDFX, was carried out on rat abdominal skin using a Franz diffusion cell. The comparative study was performed between the TEG1, Marketed NDFX formulation (Nadibact cream 1%w/w) and the plain gel of NDFX (Carbopol 1%). Figure 5 illustrates the percentage of drug permeation through rat skin. Remarkably, the optimized transethosomal gel formulation (TEG1) exhibited significantly higher drug permeation compared to the marketed cream and plain gel of NDFX i.e., 58.92%, 26.44% and 38.69%, respectively. It is ranked in the following descending order (TEG1 > plain gel > marketed cream). The high permeation rate of optimized NDFX transethosomal gel is because high flexibility of the vesicles which is attributed to the inclusion of an edge activator within the formulation. The edge activator (surfactant) provides flexibility to the vesicles, so they can deform their shape and pass through narrow obstruction of subcutaneous tissue. The ethanol present in the formulation also acts as the penetration enhancer which aids in the deep penetration of transethosomes into the underlying skin layers [12, 35].
In vitro anti-bacterial study
Zone of inhibition The cup plate method was used to evaluate the zone of inhibition of the optimized NDFX transethosome formulation against P. acness. The NDFX transethosome formulation and the marketed formulation (Nadibact Cream) exhibited noticeable zones of inhibition when compared to the blank formulation and the control, as depicted in Fig. 6. The investigation revealed that the NDFX-loaded transethosome formulation exhibited a significantly larger antimicrobial activity zone (measuring 26 ± 2 mm) compared to the marketed formulation (16 ± 1.8 mm), as well as the blank formulation and negative control. Thus, the findings demonstrated that the optimized NDFX transethosome formulation is highly responsive to P. acnes and effectively demonstrates antibacterial activity against it. The remarkable antimicrobial effect of the transethosome formulation loaded with NDFX is primarily due to the exceptional flexibility of transethosomes. This unique characteristic enables them to easily penetrate the bacterial cell wall and effectively hinder the activity of the DNA gyrase enzyme, which plays a crucial role in bacterial DNA synthesis. As a result, bacterial multiplication is effectively suppressed.
Animal study
Skin irritation study
The potential for skin irritation caused by the prepared transethosomal gel containing NDFX and the blank transethosomal gel can be visually evaluated in Fig. 7. The findings indicate that animals from both Group I (Blank gel) and Group II (NDFX transethosomal gel) did not exhibit any signs of redness or swelling until the conclusion of the seventh day. These assessments were conducted using the Draize scoring system, and the results are presented in Table 10.
P. acne-induced anti-acne study
The anti-acne efficacy of the newly developed experimental gel (NDFX-loaded transethosomal gel) was assessed in the Wistar rat model. The evaluation was conducted by measuring the percentage change in ear thickness, as depicted in Fig. 8. At the conclusion of the 14th day, the optimized transethosomal gel containing NDFX demonstrated the highest level of anti-acne efficacy. The optimized formulation has shown the least change in percentage ear thickness i.e., 22.2 ± 2.4%, followed by the marketed formulation treatment group (45.5 ± 5.2%) and plain gel treatment group (63.64 ± 6.8%). The statistical significance of the control group compared to the treatment group was examined using one-way ANOVA and Sidak’s multiple comparison tests. The disease control group exhibited a substantial percentage change in ear thickness, specifically 340 ± 12.5%, which was significantly distinguishable (p < 0.0001) from the remaining three groups. Despite the daily variation in ear thickness (from day 1 to day 14), Fig. 9 reveals a distinct pattern. In the disease control group, the thickness of the rat ear continuously increased until the 14th day. This increase was attributed to bacterial colonization and the formation of pus in the rat ear pinna. On the other hand, in Group II, Group III and Group IV, the ear thickness increased during the induction period (till the 7th day), but during the treatment period (after the 7th day), it progressively decreased due to the antibacterial properties of the formulations. The same consistent outcomes were visually depicted in the accompanying photographs (Fig. 10).
Histopathological report
Figure 11 depicts the histological changes observed in the rat ear pinna following the injection of a bacterial strain of Propionibacterium acnes and subsequent treatment with various formulations.
In the control group (non-induced), the intact epidermis, sebaceous gland and ear cartilage displayed minor congestion. However, in the case of the disease control group (untreated), the epidermis suffered mild injury, exhibiting excessive inflammatory cell infiltration, significant congestion, as well as neutrophilic and lymphocytic infiltration. Additionally, there were indications of modest folliculitis, ulceration, edema, macrophages and cutaneous granulation tissue. The group treated with the plain gel exhibited pathogenic alterations that closely resembled those observed in the disease group. These alterations included increased inflammatory cell and neutrophilic infiltration, although no edema or skin granulation tissues were identified.
Comparatively, the group treated with the standard marketed formulation (Nadibact cream) displayed mild folliculitis congestion, along with considerable neutrophilic and lymphocytic infiltration. On the other hand, the optimized transethosomal gel treated group showed an intact epidermis with modest congestion.
Post-treatment microbiological assay
The post-treatment ex vivo microbiological assay results are shown in Fig. 12. The results have demonstrated that optimized NDFX-loaded transethosomal formulation has shown potent antibacterial activity as there are fewer CFU (colony-forming units) of Propionibacterium acnes compared to the marketed formulation and the plain gel of NDFX. This might be due to the high permeability of the transethosomal vesicles into the deeper layer of the skin which allows it to retain at the subcutaneous level and inhibit bacterial colonization. The order of antimicrobial activity of the aforementioned formulations against Propionibacterium acnes is as follows:
Optimized NDFX-loaded transethosomal formulation > Marketed formulation (Nadibact cream) > Plain gel of NDFX.
Discussion
This research focused on developing Nadifloxacin-loaded transethosomes using the cold method to enhance topical delivery. A Box–Behnken experimental design successfully optimized Nadifloxacin-loaded transethosomes, with independent variables including phosphatidylcholine 90, Tween 80 and ethanol [11, 12]. Seventeen formulations were prepared, and their characterization revealed entrapment efficiency ranging from 43.64 to 81.05%, vesicle sizes between 97.12 nm and 187.34 nm, and zeta potential ranging from − 17.12 to − 34.34 mV. The optimized formulation exhibited an entrapment efficiency of 80.12% ± 0.25%, a vesicle size of 156.1 ± 2.2 nm and a zeta potential of − 33.23 ± 0.5 mV. Transmission electron microscopy (TEM) confirmed the presence of encapsulated vesicles with a sphere-like shape [21]. This optimized formulation was incorporated into gel bases, such as Carbopol 934 (1% and 2%) and HPMC K4M (2% and 3%). The characterization study demonstrated that Carbopol 934 (1%) gel exhibited desirable characteristics with a pH of 7.12, viscosity of 971 cP, spreadability of 9.2 g.cm2/sec and drug content of 92%. In vitro diffusion studies showed that the transethosomal gel with Carbopol 934 (1%) displayed superior drug diffusion after 8 h compared to Carbopol 934 (2%) and HPMC K4M (2% and 3%). Ex vivo skin permeation studies revealed that the optimized transethosomal gel demonstrated enhanced skin permeation compared to the commercially available Nadibact cream (1% w/w). The optimized Nadifloxacin-loaded transethosomal gel exhibited improved in vitro antimicrobial and in vivo anti-acne effects against Propionibacterium acnes in Wistar rats, surpassing the efficacy of the commercially available formulation. Histopathological analysis confirmed its superior anti-acne properties and better penetration capabilities compared to the plain gel and the marketed-treated group. Stability assessments over three months demonstrated the formulations' exceptional stability when stored under refrigerated conditions at 4 °C.
In conclusion, the development of Nadifloxacin-loaded transethosomes using the cold method proved effective in enhancing topical delivery for the treatment of Acne vulgaris. The optimized formulation exhibited desirable characteristics, superior skin permeation and improved anti-acne efficacy compared to the commercially available formulation, showcasing its potential as a promising approach in acne treatment.
Conclusion
Nadifloxacin-loaded transethosome formulations were successfully prepared utilizing a cold method and further optimized using a Box–Behnken design (BBD). The optimized formulation exhibited favorable characteristics, including high entrapment efficiency, vesicle size within the nanorange and desirable zeta potential. The results obtained from the ex vivo permeation study indicated that the optimized transethosomal gel facilitated enhanced permeation when compared to a commercially available formulation. The optimized transethosome formulation exhibited augmented in vitro antimicrobial activity as well as in vivo anti-acne efficacy against Propionibacterium acnes in Wistar rats, surpassing the performance of the commercially available formulation. Moreover, the histopathological report revealed that the group treated with the optimized Nadifloxacin-loaded transethosomal gel exhibited a higher rate of healing in comparison with both the plain gel and the commercially treated groups. The current investigation found NDFX-loaded transethosomal gel to be a potential alternative to standard topical formulation for the treatment of Acne.
Availability of data and materials
The data generated and analyzed during this research work are included in this article; if any excess data are required, it will be available from the corresponding author on reasonable request.
Abbreviations
- NDFX:
-
Nadifloxacin
- UDV:
-
Ultra-deformable vesicles
- P. acne :
-
Propionibacterium acne
- BBD:
-
Box–Behnken design
- PC 90:
-
Soya phosphatidylcholine 90
- VS:
-
Vesicle size
- nm:
-
Nanometer
- EE%:
-
Entrapment efficiency percentage
- ZP:
-
Zeta potential
- mV:
-
Milli-volts
- TEM:
-
Transmission electron microscopy
- PBS:
-
Phosphate buffer saline
- µg:
-
Micro-gram
- mg:
-
Milli-gram
- mL:
-
Milliliter
- min:
-
Minutes
- RPM:
-
Revolutions per minute
- w/v:
-
Weight by volume
- CDD:
-
Cumulative drug diffuse
- CDP:
-
Cumulative drug permeated
- CLA:
-
Cumulative loss added
- cps:
-
Centipoise
- MIC:
-
Minimum inhibitory concentration
- MBC:
-
Minimum bactericidal concentration
- IAEC:
-
Institutional Animal Ethics Committee
- CPCSEA:
-
Committee for the Purpose of Control and Supervision of Experiments on Animals
References
Anjum S, Tabasum A, Manzoor F, Faisal MU (2021) Concept of Busoore Labaniya (Acne vulgaris) and its management in light of unani system of medicine. J Drug Deliv Ther 11(5-S):159–163. https://doi.org/10.22270/jddt.v11i5-s.5081
Tamanna SH (2021) Nanotechnology: a novel approach for treatment of skin disorder. J Drug Deliv Ther 11(4-S):271–277. https://doi.org/10.22270/jddt.v11i4-s.4945
Bowe WP, Glick JB, Shalita AR (2012) Solodyn and updates on topical and oral therapies for acne. Curr Dermatol Rep 1(3):97–107. https://doi.org/10.1007/s13671-012-0014-x
Cheng Y, Qu H, Ma M, Xu Z, Xu P, Fang Y, Xu T (2007) Polyamidoamine (PAMAM) dendrimers as biocompatible carriers of quinolone antimicrobials: an in vitro study. Eur J Med Chem 42(7):1032–1038. https://doi.org/10.1016/j.ejmech.2006.12.035
Shinde U, Pokharkar S, Modani S (2012) Design and evaluation of microemulsion gel system of Nadifloxacin. Indian J Pharm Sci 74(3):237–247. https://doi.org/10.4103/0250-474x.106066
Kumar A, Agarwal SP, Ahuja A, Ali J, Choudhry R, Baboota S (2011) Preparation, characterization, and in vitro antimicrobial assessment of nanocarrier based formulation of nadifloxacin for acne treatment. Pharmazie 66(2):111–114. https://doi.org/10.1691/ph.2011.0227
Ascenso A, Raposo S, Batista C, Cardoso P, Mendes T, Praça FG, Bentley MVLB, Simões S (2015) Development, characterization, and skin delivery studies of related ultradeformable vesicles: transfersomes, ethosomes, and transethosomes. Int J Nanomed 10:5837–5851. https://doi.org/10.2147/IJN.S86186
Vinod KR, Kumar MS, Anbazhagan S, Sandhya S, Saikumar P, Rohit RT, Banji D (2012) Critical issues related to transfersomes—novel vesicular system. Acta Sci Pol Technol Aliment 11(1):67–82
Kesharwani R, Patel DK, Sachan A, Kumar V, Mazumdar B, Assam D (2015) Ethosomes: a novel approch for transdermal and tropical drug delivery. World J Pharm Pharm Sci 4(06):348–359. https://doi.org/10.5958/2321-5844.2015.00003.5
Bajaj KJ, Parab BS, Shidhaye SS (2021) Nano-transethosomes: a novel tool for drug delivery through skin. Indian J Pharm Educ Res 55(1):s1–s10. https://doi.org/10.5530/ijper.55.1s.33
Abdulbaqi IM, Darwis Y, Assi RA, Khan NAK (2018) Transethosomal gels as carriers for the transdermal delivery of colchicine: statistical optimization, characterization, and ex vivo evaluation. Drug Des Devel Ther 12:795–813. https://doi.org/10.2147/DDDT.S158018
Adnan M, Afzal O, Altamimi ASA, Alamri MA, Haider T, Faheem HM (2023) Development and optimization of transethosomal gel of apigenin for topical delivery: In-vitro, ex-vivo and cell line assessment. Int J Pharm 631:122506. https://doi.org/10.1016/j.ijpharm.2022.122506
Shamma RN, Elsayed I (2013) Transfersomal lyophilized gel of buspirone HCl: formulation, evaluation and statistical optimization. J Liposome Res 23(3):244–254. https://doi.org/10.3109/08982104.2013.801489
Touitou E, Dayan N, Bergelson L, Godin B, Eliaz M (2000) Ethosomes-novel vesicular carriers for enhanced delivery: characterization and skin penetration properties. J Control Release 65:403–418. https://doi.org/10.1016/s0168-3659(99)00222-9
Zaki RM, Seshadri VD, Mutayran AS, Elsawaf LA, Hamad AM, Almurshedi AS, Yusif RM, Said M (2022) Wound healing efficacy of rosuvastatin transethosomal gel, I optimal optimization, histological and in vivo. Eval Pharm 14(11):2521. https://doi.org/10.3390/pharmaceutics14112521
Daood NM, Jassim ZE, Gareeb MM, Zeki H (2019) Studying the effect of different gelling agent on the preparation and characterization of metronidazole as topical emulgel. Asian J Pharm Clin Res 12:571–577
Abdellatif AAH, Aldosari BN, Al-Subaiyel A, Alhaddad A, Samman WA, Eleraky NE, Elnaggar MG, Barakat H, Tawfeek HM (2023) Transethosomal gel for the topical delivery of celecoxib: formulation and estimation of skin cancer progression. Pharmaceutics 15(1):22. https://doi.org/10.3390/pharmaceutics15010022
Naga Sravan Kumar Varma V, Maheshwari PV, Navya M, Reddy SC, Shivakumar HG, Gowda DV (2014) Calcipotriol delivery into the skin as emulgel for effective permeation. Saudi Pharm J 22(6):591–599. https://doi.org/10.1016/j.jsps.2014.02.007
Rahangdale M, Pandey P (2021) Development and characterization of apremilast transethosomal gel for transdermal delivery. Int J Pharm Sci Nanotechnol 14(3):5508–5518. https://doi.org/10.37285/ijpsn.2021.14.3.8
Hassan AS, Hofni A, Abourehab MA, Abdel-Rahman IA (2023) Ginger extract-loaded transethosomes for effective transdermal permeation and anti-inflammation in rat model. Int J Nanomed 18:1259–1280. https://doi.org/10.2147/ijn.s400604
Adin SN, Gupta I, Rashid MA, Alhamhoom Y, Aqil M, Mujeeb M (2023) Nanotransethosomes for enhanced transdermal delivery of mangiferin against rheumatoid arthritis: formulation, characterization, in vivo pharmacokinetic and pharmacodynamic evaluation. Drug Deliv 30(1):2173338. https://doi.org/10.1080/10717544.2023.2173338
Moolakkadath T, Aqil M, Ahad A, Imam SS, Iqbal B, Sultana Y, Mujeeb M, Iqbal Z (2018) Development of transethosomes formulation for dermal fisetin delivery: Box–Behnken design, optimization, in vitro skin penetration, vesicles–skin interaction and dermatokinetic studies. Artif Cells Nanomed Biotechnol 46(sup2):755–765. https://doi.org/10.1080/21691401.2018.1469025
Okamoto K, Ikeda F, Kanayama S, Nakajima A, Matsumoto T, Ishii R, Umehara M, Gotoh N, Hayashi N, Iyoda T et al (2016) In vitro antimicrobial activity of benzoyl peroxide against Propionibacterium acnes assessed by a novel susceptibility testing method. J Infect Chemother 22(6):426–429. https://doi.org/10.1016/j.jiac.2015.12.010
Apriani E, Rosana Y, Iskandarsyah I (2019) Formulation, characterization, and in vitro testing of azelaic acid ethosome-based cream against Propionibacterium acnes for the treatment of acne. J Adv Pharm Technol Res 10(2):75–80. https://doi.org/10.4103/japtr.JAPTR_289_18
Ahmed TA, Alzahrani MM, Sirwi A, Alhakamy NA (2021) The antifungal and ocular permeation of ketoconazole from ophthalmic formulations containing trans-ethosomes nanoparticles. Pharmaceutics 13(2):1–24. https://doi.org/10.3390/pharmaceutics13020151
Khan MA, Pandit J, Sultana Y, Sultana S, Ali A, Aqil M, Chauhan M (2015) Novel carbopol-based transfersomal gel of 5-fluorouracil for skin cancer treatment: in vitro characterization and in vivo study. Drug Deliv 22(6):795–802. https://doi.org/10.3109/10717544.2014.902146
Julianti E, Rajah KK, Fidrianny I (2017) Antibacterial activity of ethanolic extract of Cinnamon bark, honey, and their combination effects against acne-causing bacteria. Sci Pharm 85(2):19. https://doi.org/10.3390/scipharm85020019
Najafi-Taher R, Ghaemi B, Amani A (2018) Delivery of adapalene using a novel topical gel based on tea tree oil nano-emulsion: permeation, antibacterial and safety assessments. Eur J Pharm Sci 120:142–151. https://doi.org/10.1016/j.ejps.2018.04.029
De Young LM, Young JM, Ballaron SJ, Spires DA, Puhvel SM (1984) Intradermal injection of Propionibacterium acnes: a model of inflammation relevant to acne. J Investig Dermatol 83(5):394–398. https://doi.org/10.1111/1523-1747.ep12264715
Gull A, Ahmed S, Ahmad FJ, Nagaich U, Chandra A (2020) Hydrogel thickened microemulsion; a local cargo for the co-delivery of cinnamaldehyde and berberine to treat Acne vulgaris. J Drug Deliv Sci Technol 58:101835. https://doi.org/10.1016/j.jddst.2020.101835
El Zaafarany GM, Awad GAS, Holayel SM, Mortada ND (2010) Role of edge activators and surface charge in developing ultradeformable vesicles with enhanced skin delivery. Int J Pharm 397(1–2):164–172. https://doi.org/10.1016/j.ijpharm.2010.06.034
Ahad A, Aqil M, Kohli K, Sultana Y, Mujeeb M (2013) Enhanced transdermal delivery of an anti-hypertensive agent via nanoethosomes: statistical optimization, characterization and pharmacokinetic assessment. Int J Pharm 443(1–2):26–38. https://doi.org/10.1016/j.ijpharm.2013.01.011
Ogiso T, Yamaguchi T, Iwaki M, Tanino T, Miyake Y (2001) Effect of positively and negatively charged liposomes on skin permeation of drugs. J Drug Target 9(1):49–59. https://doi.org/10.3109/10611860108995632
Gadad AP, Patil AS, Singh Y, Dandagi PM, Bolmal UB, Basu A (2020) Development and evaluation of flurbiprofen loaded transethosomes to improve transdermal delivery. Indian J Pharm Educ Res 54(4):954–962. https://doi.org/10.5530/ijper.54.4.189
Albash R, Abdelbary AA, Refai H, El-Nabarawi MA (2019) Use of transethosomes for enhancing the transdermal delivery of olmesartan medoxomil: in vitro, ex vivo, and in vivo evaluation. Int J Nanomed 14:1953–1968. https://doi.org/10.2147/IJN.S196771
Acknowledgements
We gratefully acknowledge Wockhardt Research Centre, Aurangabad, India, and Lipidome Lifesciences, Ahmedabad, India, for providing gift samples of NDFX and Soya Phosphatidylcholine-90, respectively. We would also like to acknowledge Department of Pharmaceutics, KLE College of Pharmacy, KLE Academy of Higher Education and Research (KAHER), Belagavi, and Dr. Prabhakar Kore Basic Science Research Center (BSRC) for providing the facility to perform the research study.
Funding
No funding was received for this research work.
Author information
Authors and Affiliations
Contributions
Author SP performed all the above research work and prepared the complete manuscript, Author PD and TK guided and monitored the research activities, Author PB and VK guided in performing the animal experiment and antimicrobial study, respectively, Author SH contributed in final drafting of manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Necessary authorization and endorsement were obtained from the Institutional Animal Ethics Committee (IAEC) and the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), under the Reg. No. 221/Po/Re/S/2000/CPCSEA, during their meeting on 03/12/2022.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Patil, S., Dandagi, P.M., Kazi, T. et al. A DoE-based development and characterization of Nadifloxacin-loaded transethosomal gel for the treatment of Acne vulgaris. Futur J Pharm Sci 10, 46 (2024). https://doi.org/10.1186/s43094-024-00616-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43094-024-00616-2