Abstract
The formation of mineral scale has been a major constraint in the oilfield operations as it leads to numerous flow assurance issues. Scale deposition in the formation and production tubing can restrict the flow of hydrocarbon and interferes with the running and operation of downhole equipment. Scale inhibition squeeze treatment is one of the most common form of scale prevention. Although current squeeze treatment is the optimal way to prevent scale from depositing, it is still lack in certain aspect such as adsorption ability and retention time within the rock formation. This paper presents promising advantages of engaging nanotechnology to enhance current scale inhibition treatment. Experimental studies were carried out to examine the potential benefits of using graphene oxide and carbon nanotubes to increase the adsorption of conventional scale inhibitor, ethylenediaminetetraacetic acid (EDTA) on rock formation in a process called nano-carbon enhanced squeeze treatment (NCEST). This process involves treating the rock surface in the near wellbore region with nanomaterials that allow better adsorption capacity of scale inhibitor. Analysis testing using various techniques including field-emission scanning electron microscopy, energy-dispersive X-ray and ultraviolet–visible spectrophotometer were conducted to study the adsorption, retention and bonding of the scale inhibitor with nanomaterials and rock. NCEST technique was observed to significantly increase the adsorption of EDTA on rock sample treated with nanomaterials with a maximum adsorption of 180 mg/g compared to 51 mg/g on rock sample without nanomaterials treatment. In terms of cost–benefit, it is estimated to have significant reduction in operating expenses (up to 50%) after implementing the NCEST technique compared to that of conventional squeeze treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
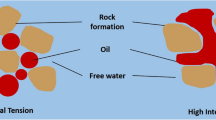
The formation of mineral scale associated with production of hydrocarbon has been a major strain in oilfield operation. Relative to the nature of the scale and formation fluid composition, scale deposition can take place within a reservoir which causes formation damage, or in the production system where blockage can cause severe operational problems and interfere with the running and operation of downhole equipment. In most cases, the most common type of scale found in wells is caused by the formation of sulfate and carbonate scales of calcium and strontium. Many oil and gas fields use sea water or brine injection for primary oil recovery or pressure maintenance. This brine is the primary cause of calcite and sulfate scales deposition. Because of their relative hardness and low solubility, removal of scale will cost a lot of money to the company. This is where a proactive measure such as the ‘squeeze’ treatment is needed to prevent the precipitation of scale entirely (Moghadasi et al. 2007).
Scale inhibition is the method of preventing the precipitation of scale by injecting chemical inhibitor into the formation known as the ‘squeeze’ treatment. In a conventional squeeze treatment, acid phosphonate inhibitors are commonly used for downhole application in many oil reservoirs around the world (Jordan et al. 1994). The limitation of current scale inhibitor used is the precipitation of acid phosphonate near the entrance of formation results in limited reservoir protection distance near the well bore. Apart from that, during the precipitation squeeze, only little or negligible amount of phosphonate inhibitor can be retained and slowly released from the formation. This results in a large fraction of the inhibitor flowing back within a few days, leaving an extremely low value in the reservoir that is not sufficient for effective scale inhibition (Shen et al. 2008). Therefore, numerous studies have been carried out to enhance the adsorption level of inhibitor onto the formation to ensure a successful and effective scale inhibition treatment in the oilfields.
In light of the recent interest in nanotechnology application in the oil and gas industry, several researchers have sparked their interest in adopting this technology for scale inhibition purpose using nanomaterials (Ogolo et al. 2012; Kumar et al. 2012; Tian et al. 2013). Recent study explored the potential benefit of using carbon nanotubes (CNTs) to enhance squeeze treatment lifetime by increasing the adsorption of scale inhibitor within the formation. Simple adsorption tests were carried out to evaluate the adsorption of polyphosphinocarboxylic acid (PPCA) scale inhibitor on CNTs. The preliminary result of the study shows a promising outcome where the adsorption and retention of scale inhibitor within the formation demonstrate a dramatic increase (Ghorbani et al. 2014). The purpose of this paper is to explore other applications of nanomaterials such as graphene oxide (GO) which have a similar characteristic as CNTs in improving the scale inhibitor squeeze treatment lifetime. This work optimized the nanomaterials in enhancing conventional scale inhibitor (EDTA) adsorption and retention ability on the formation.
The main objective of this study is to develop a new methodology that can further expand the scale inhibitor squeeze lifetime using graphene oxide (GO) and carbon nanotubes (CNTs). To achieve this, the following supporting objectives were proposed:
Optimizing GO and CNTs as the nanomaterial to enhance the adsorption of conventional scale inhibitor (EDTA) into the rock formation which in turn will expand squeeze treatment lifetime.
To facilitate better adsorption of scale inhibitor onto the rock surface by modifying the rock properties to create a strong covalent bond with nanomaterials.
To increase inhibitor retention time at the scaling site in order to reduce the tendency of large fraction of scale inhibitor flowing back to the surface during production.
Literature review
Introduction to scale
Scale is an inorganic mineral deposit formed as a result of supersaturation at wellbore condition or commingling of incompatible fluids. Saturated brines form precipitates when its mineral equilibrium concentration are exceeded. This may be due to increased mineral concentration, change in temperature, change in pressure or pH, or mixing of incompatible waters. Scale in the oilfields can be deposited by direct precipitation of produced water from the reservoir, or as the byproduct of formation water becoming oversaturated with scale minerals when two incompatible water mixes (Tantayakom et al. 2005). When water is injected for enhanced oil recovery into well that have water production, there are chances for precipitation of scale (Crabtree et al. 1999). Some of the common types of scales are calcium carbonate (CaCO3), barium sulfate (BaSO4), and calcium sulfate (CaSO4).
Scale inhibition
While there are many ways to remove scale deposition inside the tubing and formation, scale removal does not offer a permanent solution as scale can start to build up again in a few years. That is why scale inhibition treatment is important as it can control and prevent further deposition of scale inside the tubing or formation. Scale inhibitor is a specially designed chemical that is injected or installed in fluid flow systems to slow down or prevent precipitation and aggregation of scale on the walls of the system. In ideal cases, proactive measure to prevent scale can be taken by injecting scale inhibitor prior well production in wells that have a tendency to produce scale. Nevertheless, scale inhibitor can also be injected or squeezed after scale already built up as long as the existing scale is removed first from the tubing or formation. This is to ensure the usefulness of the scale inhibitor to prevent further deposition. Scale inhibitor can also be injected routinely throughout the life of a well (Kelland 2009).
Nanotechnology application for scale inhibition
From the conventional squeeze treatment, it is observed that the apparent lack of suitable surfaces available for adsorption is the main flaw in this method. In sandstones and quartz, its main constituent minerals have a very low ability to adsorb inhibitor. Therefore, research has been focused on using a nanomaterial known as carbon nanotubes (CNTs) to enhance the available sites for scale inhibitor adsorption within the near wellbore region (Kumar et al. 2012). CNTs demonstrate extraordinary properties that make them attractive in mitigating well bore problems. They are stable at high temperature and have a high Young’s modulus that enables them to withstand the high pressure and temperatures in the reservoir. CNTs abundance of carbon atoms can afford many active sites for functionalization by scale inhibitors, and their high specific surface area can provide extensive physical adsorption of inhibitors (Ghorbani et al. 2014).
The purpose of this paper is to explore this method more thoroughly by experimenting with another type of nanomaterial which is graphene. Graphene is one of the allotropes of carbon that has a single atom thickness and a two-dimensional (2D) sheet-like structure as shown in Fig. 1. The carbon atoms in graphene are arranged in a hexagonal-shaped pattern where each of the carbon atom is bonded to four other carbon atoms and contains a delocalized electron. Graphene have a very similar atomic structure and properties as the carbon nanotubes so it can be used to associate CNTs in enhancing scale inhibitor adsorption to the formation by ensuring the adsorption agent act in all dimension as a 2D sheets and dispersed uniformly throughout the solution. Homogenous dispersion of the nanomaterials is the key factor that increases the potential functionality of the used material (EAG Laboratories Inc 2017).
Graphene (left) and carbon nanotubes (right) structure (Nascentes et al. 2001)
Ncest methodology
The nano-carbon enhanced squeeze treatment (NCEST) methodology is a newly developed methodology that focuses on enhancing the adsorption of scale inhibitors onto the rock surface to increase the squeeze treatment lifetime. This is done by modifying the chemistry of the rock surface using a binder known as cellulose. There are four major steps that were performed to achieve the objective above. The steps are explained briefly as follows:
- (a)
Deposition of a binder on a surface of a geological formation;
- (b)
Delivering carbon-based nanomaterial to the surface of the geological formation to allow adherence between the nanomaterial and the binder by chemical interaction, wherein the nanomaterial provides one or more adsorption sites for scale inhibitor;
- (c)
Injecting an amount of scale inhibitor in the modified geological formation surface for the inhibitor to adsorb by the nanomaterial; and
- (d)
Inhibit scale growth in the geological formation by sustained release of the amount of scale inhibitor from the nanomaterial into the geological formation (Ghorbani et al. 2014).
This methodology is divided into three experiments, namely Experiment A, B, and C. Experiment A tests out the feasibility of employing nanomaterials to enhance scale inhibitor squeeze treatment by conducting adsorption test in static condition, whereas Experiment B and C test their feasibility in dynamic condition by carrying out coreflood test.
Experiment A: adsorption test
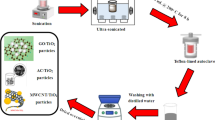
The first part of this NCEST methodology involves examining the adsorption level of scale inhibitor on and from the nanomaterials. This experiment is the most important part in this study as it determines whether the squeeze lifetime can be expanded from the result of adsorption of inhibitor in the formation. EDTA scale inhibitor is applied in this treatment to replace the PPCA used in the experiment work by Ghorbani et al. (2017). EDTA is one of the conventional scale inhibitor commonly used in the industry. Adsorption test was conducted both in static and dynamic condition. For static condition, the experiment was carried out by simply stirring the nanomaterials with EDTA for 24 h in ambient temperature and pressure.
Materials, apparatus, and equipment
Carbon nanotubes were supplied in powder form by Carbon Nano-material Technology Co. Ltd. The supplied CNTs are multi-walled carbon nanotubes with ~ 15 nm diameter and ~ 5 µm length. Reduced graphene oxide was provided in aqueous solution stabilized with poly (sodium 4-styrenesulfonate) dispersion from Sigma-Aldrich. Calcium chloride dihydrate (CaCl2·2H2O) and ethylene-diamine tetraacetic acid (EDTA) salt are taken and used as provided from laboratory. Ultraviolet–visible (UV–Vis) equipment is used to characterize the concentration of EDTA before and after the adsorption test. The solution will be filtered using PVDF syringe filter with pore size of 0.45 µm supplied from Ricco Labstore.
Experimental procedure
-
i.
About 300 ml of 0.05 M EDTA in distilled water (DW) was prepared. About 4-ml sample of EDTA in DW solution was taken to be used as initial concentration.
-
ii.
About 300 ml of brine solution was prepared by diluting 1147.8 mg of calcium chloride dihydrate (CaCl2·2H2O) salt in 300 ml DW. About 0.05-M EDTA was prepared in the brine solution. About 4-ml sample of EDTA in brine solution was taken as initial concentration.
-
iii.
About ml of 0.05 M EDTA in water/brine solution was poured into six beakers.
-
iv.
Desired amount of nanomaterial was added into each of the beaker and magnetically stirred using magnetic stirrer.
-
v.
After 1 h, 4 ml of the solution was filtered using 0.45 µm syringe filter for UV–Vis measurement.
-
vi.
The solution was then left to be stirred for another 23 h. After 24 h, 3 ml of the solution is taken and filtered using syringe filter for UV–Vis measurement.
Experiment B: dispersion of nanomaterials
Nanoparticles have the tendency of being conveniently aggregate/agglomerate/coalescence and quickly removed through stationary porous media (Shen et al. 2008). Therefore, dispersing the nanomaterial is very important to avoid plugging of the wellbore during injection of the nanofluid. Several dispersing agents in the form of solvents have been identified as being principally good at dispersing carbon-based nanomaterials such as GO and CNTs. Among the solvents are N-methyl-2-pyrrolidone (NMP), sodium dodecyl sulfate (SDS), dimethyl sulfoxide (DMSO), and N,N-dimethylformamide (DMF). In this experiment, chemical sodium dodecyl sulfate (SDS) is the candidate for nanomaterial dispersion study.
Apart from studying on the nanomaterial dispersion, this experiment also acts as preparation for the next experiment, which is the coreflood test. In order to inject the nanomaterial fluid into the core, the nanomaterial must be in the form of stabilized nanofluid solution to ensure that homogenous dispersion can be achieved.
Materials, apparatus, and equipment
Carbon nanotubes (CNTs) and graphene oxide are supplied from previous mentioned seller. Sodium dodecyl sulfate (SDS) salt is provided from R&M Chemicals. Ultrasonic bath was used to disperse the nanomaterial by the process of sonication. In this experiment, only one nanomaterial is dispersed due to equipment and time constraint. The type of nanomaterial (GO or CNTs) that has been used for this experiment is optimized from experiment A.
Experimental procedure
-
i.
About 1000 ml of 2% SDS dispersant was prepared by diluting sodium dodecyl sulfate (SDS) powder in distilled water (DW).
-
ii.
Three beakers were filled with 200 ml of the SDS solution each:
-
iii.
Desired amount of nanomaterial was added into the SDS solution followed by putting the beaker in an ultrasonic bath for 2 h. The solution was then left static for 24 h to precipitate the un-functionalized nanoparticles to the bottom of the beaker.
-
iv.
Only the upper suspension (top half) was used in the next experiment to ensure that the nanomaterials used are well dispersed and remove the possibility of agglomerated nanoparticles on the substrate (See Table 1).
Table 1 Dispersion test preparation
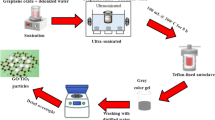
Experiment C: coreflooding test
This experiment was carried out to test the feasibility of employing nanomaterials to enhance scale inhibitor squeeze treatment in dynamic condition. Coreflooding is a laboratory test that is aimed to simulate the NCEST methodology process in real reservoir condition. Ultimately, the result from this experiment was used to evaluate its practicality to be carried out in the real field.
Materials, apparatus, and equipment
This experiment was conducted using Barea sandstone plug-sized core samples of varied porosity and permeability to represent rock formation. The coreflood test is performed using BPS-805 benchtop liquid permeameter at ambient temperature with the flow rate of 1 ml/min. Before the core plugs were sent for coreflooding, it needs to go through a series of preparation to determine its properties. The length, diameter, and weight of the core plugs are measured using caliper and weighing balance, while its porosity, permeability, and pore volume are calculated using POROPERM COVAL 30 equipment. In the coreflooding experiment, several solutions were injected into the core samples. The solutions include ethyl cellulose, EDTA scale inhibitor, and distilled water.
Solution preparation
From the result of POROPERM analysis, the pore volume for each core plugs was known. This is useful in preparing the solution to be injected in the coreflooding test as the unit used in terms of pore volume (PV).
- i.
The amount of solution in cc for 1 PV was calculated from POROPERM result.
- ii.
Brine: 10 PV of brine solution was prepared by adding 3.812 g/l sodium chloride (NaCl) and 3.826 g/l calcium chloride dihydrate (CaCl2·2H2O) powder in distilled water.
- iii.
EDTA and DW: 10 PV of 1775 ppm EDTA and DW solution was prepared by adding EDTA salt into distilled water and stirred using magnetic stirrer.
Experimental procedure: coreflooding
-
i.
The coreflood test was performed at room temperature with the flow rate of 1 ml/min.
-
ii.
About 10 PV of cellulose was injected into the core followed by shutting the core for 1 h.
-
iii.
About 10 PV of dispersed nanomaterial solution was subsequently injected into the core, and the core is shut for 24 h.
-
iv.
The core was afterward rinsed with 10 PV of brine solution.
-
v.
About 10 PV of EDTA in DW solution was then injected into the core, and effluent samples were taken for UV–Vis measurement.
-
vi.
The core was then shut in for a further 24 h.
-
vii.
Post-flush was begun using DW and effluent again sample for UV–Vis measurement.
-
viii.
Core sample was taken and sent for FESEM measurement.
Experimental procedure: baseline core flooding
-
i.
About 10 PV of EDTA and DW solution was injected into the core, and effluent samples were taken for UV–Vis measurement.
-
ii.
The core was shut for 24 h to enable the EDTA to adsorb on the rock.
-
iii.
The core was afterward rinsed with 10 PV of brine solution.
-
iv.
Background solution (DW) is pumped into the core, and effluent samples are taken for UV–Vis measurement.
-
v.
Core sample was taken and sent for FESEM measurement.
Results and discussion
Stage 1: adsorption test
From the UV–Vis spectrophotometry reading, the value of liquid absorbance can be determined. To quantify the adsorption in terms of initial and final concentration of the solution, the absorbance value can be converted into concentration by using Beer’s law. Beer’s law states that a substance’s concentration and its absorbance are directly proportional. Concentration of an unknown solution can be calculated by preparing a standard solution with known concentration. The standard can range from the smallest concentration to the maximum concentration the sample can achieve. In this experiment, the initial concentration of EDTA in DW/brine was set at 0.05 M. After the adsorption experiment, its concentration is expected to decrease. Hence, the concentration of standard that was prepared is EDTA in DW/brine of 0.01 M, 0.02 M, 0.03 M, 0.04 M, 0.05 M, and 0.06 M.
After the standard solution is prepared and tested using UV–Vis equipment, graph of absorbance vs concentration was drawn. The absorbance value must be taken at the same wavelength as a control. From the UV–Vis reading, the highest value of absorbance was recorded at the range of ~ 200 nm. Therefore, absorbance values at 200 nm wavelength were taken and used in this experiment. Graphing will validate the assumption of Beer’s Law by looking for a straight-line relationship of the data. Figure 2 below shows the graph of best fit for standard solutions:
From the graph, concentration of an unknown sample can be determined from its absorbance value by interpolation. This is done by drawing a horizontal line from the y-axis at samples’ absorbance value until it crosses the best fit line. Then, a vertical line is drawn from that point to the x-axis. The x-value at this point is the concentration of the sample. The second method that can be used to determine the concentration is by simply calculating the value using equation of the straight line in the graph as shown in (1). Based on the samples’ absorbance values measured by UV–Vis equipment, bar charts of concentration of EDTA in DW/brine against types of nanomaterials are constructed and shown in Figs. 3 and 4.
The huge difference in concentration value of EDTA before and after the stirring process shows that a significant amount of EDTA inhibitor has been adsorbed onto the nanomaterials. Quick adsorption of EDTA on the nanomaterials can also be observed from this experiment as only 1 h is needed for the EDTA to be effectively adsorbed. From the initial and final concentration of EDTA in DW/brine for different nanomaterials, the concentration reduction in EDTA in varying nanomaterials was calculated to better differentiate which nanomaterials manage to reduce the highest concentration of EDTA over an hour. Apart from that, the adsorption capacity, q, of the nanomaterials is also calculated using equation below and its results are tabulated in Table 2.
where q = adsorption capacity (mg/g), Ci = initial concentration (mg/l), Cf = final concentration (mg/l), V = volume of adsorbent used (l), and W = weight of the adsorbent (g).
Figure 5 and Table 2 show that the best performing nanomaterials is graphene oxide (GO). This can be clearly seen as GO gives the highest reduction in EDTA concentration compared to CNTs and CNTs combined with GO in both distilled water and brine as the background solution. This may be due to the structural properties of the graphene itself. Since graphene have a more sheet-like structure and CNTs are tube-shaped, graphene particles have lower tendency to agglomerates between each other. CNTs on the other hand are more prone to agglomeration due to the van der Waals force acting between them. Agglomeration of the nanoparticles has lessened its efficiency as an adsorbent as the active sites for adsorption to take place reduces. Graphene also has a larger surface area for the scale inhibitor to be adsorbed compared to CNTs.
Apart from the different types of nanomaterials, type of background solution used also plays a significant role in enhancing the adsorption of scale inhibitor onto the nanomaterials. In this experiment, two types of background solution were used which are distilled water and brine, which is a solution of calcium chloride dihydrate (CaCl2·2H2O). The purpose of using brine is to investigate the adsorption ability of EDTA scale inhibitor in the presence of calcium ions, Ca2+. From results shown in Fig. 6, it was observed that the adsorption of EDTA in brine as the background solution is consistently higher than in distilled water. This indicates that the presence of calcium ions can enhance the adsorption of EDTA by providing two free electrons to bond with the EDTA and nanomaterials.
From this static adsorption test (Experiment A), it can be concluded that GO is the best nanomaterials to be used as an adsorbent to enhance scale inhibitor adsorption ability. Because of this, GO was used in the next stage of NCEST methodology which is the dynamic adsorption test to examine its ability to bond with the rock surface and its adeptness to adsorb scale inhibitor as well as in the static adsorption test.
Stage 2: coreflooding test
After necessary injection process is performed at a flowrate of 1 ml/min, the effluent samples from the coreflood equipment are taken and sent for UV–Vis measurement. To measure the effectiveness of using nanomaterials to enhance the adsorption of scale inhibitor onto the formation, the experiment was conducted with two core samples which are with nanomaterial injection and without nanomaterials (baseline). The results of these two core samples were compared, and further analysis was carried out. To analyze the concentration of samples before and after the experiment, the process goes through the same flow as in Experiment A. The absorbance value at 200 nm wavelength was taken and interpolated in the calibration curve to get its concentration value. After that, a graph of concentration against core samples was drawn.
From the result, there is a huge difference in concentration for core sample treated with nanomaterials compared to core sample without any nanomaterials injection. This shows that more adsorption occurs in the rock sample that was treated with nanomaterials. This is because the presence of nanomaterials provides and increases active sites for the adsorption of scale inhibitor on the substrate. Low value of final concentration of EDTA also implies that there is a good retention of EDTA on the formation. This will benefit in the long run as the squeeze treatment lifetime can last longer and reduces the cost of redoing scale inhibition treatment over a short period of interval. The adsorption capacity of EDTA for both cases is calculated and shown in Fig. 7 and Table 3.
Although in this experiment graphene oxide (GO) is used as the nanomaterial, its adsorption value is not the same as in the static adsorption test in Experiment A. In the static adsorption test, the adsorption capacity of EDTA on GO is approximately ~ 590 mg/g, while in this test, the value is at 180 mg/g. This is because in dynamic adsorption test, not all of the surface area of GO is in contact with the EDTA as it is attached on the rock. Therefore, the value may be much lower than in the adsorption test, where almost its entire surface is in contact with EDTA. Since the coreflooding test is the experiment that can best simulate the squeeze treatment process in reservoir condition, its adsorption value is more realistic.
Field-emission scanning electron microscopy (FESEM) analysis
After finished with the coreflooding test, a small fraction of the core samples was sent for FESEM analysis. This analysis was done to examine the topographical and elemental information of nanoparticles and EDTA on the rock surface at very high magnification. Images in Fig. 8 show the graphene structure on the rock surface, which is the hard evidence of nanomaterials attachment on the rock surface. Apart from the images, the machine is also equipped with an energy-dispersive X-ray (EDX) system that analyzes the elemental content on the rock. Result from EDX can be pre-programmed to show the atomic percentage of desired elements.
From the chemical formula of EDTA (C10H16N2O8), the elements that make up this acid are carbon, nitrogen, and oxygen as shown in Table 4. Given results from the EDX analysis shows that the weight percentage of these three elements exists abundantly after the injection of EDTA on both baseline and with nanomaterials core samples. This indicates that the EDTA scale inhibitor has adsorbed onto the rock formation. However, the increment is higher in core sample that was treated with nanomaterial GO. This further supports the result from coreflooding test, which states that the adsorption of EDTA onto rock formation treated with nanomaterials is higher than on core sample that is without any nanomaterial.
Conclusion
The main objective of developing new methodology of optimized nanomaterial, namely graphene oxide (GO) and carbon nanotubes (CNTs) in expanding scale inhibitor squeeze lifetime was achieved successfully. Based on the outcome from this study, nanomaterials have proven to be effective as an agent to increase and facilitate the adsorption of scale inhibitor onto the rock surface. In this study, the authors managed to investigate the performance of different types of nanomaterials. It was found that graphene oxide (GO) gives the optimal performance in enhancing squeeze treatment lifetime due to its structural properties compared to carbon nanotubes (CNTs). Other than that, it was also proven that treating the rock surface with nanomaterials can significantly increase the adsorption rate of EDTA scale inhibitor on the formation rock. Adsorption rate of EDTA on core sample treated with graphene oxide is at 180 mg/g, while core sample with no nanomaterials treatment is only at 51 mg/g.
The results show a promising outcome in optimizing nano-based materials to facilitate better adsorption of scale inhibitor. Further development of this research can include:
Investigating the optimum dispersant concentration of different nanomaterials to achieve highest dispersion,
Studying the desorption rate of EDTA from nanomaterials to ensure that the scale inhibitor can desorbed back into the produced water at ideal concentration,
Employing NCEST methodology on different types of carbon-based nanomaterials or scale inhibitor chemical as different types of scale inhibitor may have varying suitability with the nanomaterials.
Recommendation
Although the proven concept was encouraging, there are still several areas in the methodology that needs to be extensively studied for industrial use. This can be done by performing experimental work that can simulate an environment that is as close as the real reservoir condition.
References
Crabtree M, Eslinger D, Fletcher P, Miller M, Johnson A, King G (1999) Fighting scale-removal and prevention. Oilfield Rev 11:30–45
EAG Laboratories Inc (2017) Ultraviolet–Visible spectroscopy (UV–Vis). Retrieved on 24th October 2017 from https://www.eag.com/uv-vis-spectroscopy/
Ghorbani N, Wilson MC, Kapur N, Fleming N, Neville A (2014) Carbon nanotubes: a new methodology for enhanced squeeze lifetime CNTs. In: SPE international oilfield scale conference and exhibition. https://doi.org/10.2118/169763-ms
Ghorbani N, Wilson M, Kapur N, Fleming N, Tjomsland T, Neville A (2017) Adsorption of polyphosphinocarboxylic acid (PPCA) scale inhibitor on carbon nanotubes (CNTs): a prospective method for enhanced oilfield scale prevention. J Petrol Sci Eng 150:305–311. https://doi.org/10.1016/j.petrol.2016.12.016
Jordan M, Sorbie K, Ping J, Yuan M, Todd A, Hourston K (1994) Phosphonate scale inhibitor adsorption/desorption and the potential for formation damage in reconditioned field core. In: Proceedings of SPE formation damage control symposium. https://doi.org/10.2523/27389-ms
Kelland MA (2009) Production chemicals for the oil and gas industry. CRC Press, Boca Raton
Kumar D, Chishti SS, Rai A, Patwardhan SD (2012) scale inhibition using nano-silica particles. In: SPE middle east health, safety, security, and environment conference and exhibition. https://doi.org/10.2118/149321-ms
Moghadasi J, Jamialahmadi M, Müller-Steinhagen H, Sharif A (2007) Scale deposits in porous media and their removal by EDTA injection. In: Proceedings of 7th international conference on heat exchanger fouling and cleaning—challenges and opportunities
Nascentes CC et al (2001) Use of ultrasonic baths for analytical applications: a new approach for optimization conditions. J Braz Chem Soc 12(1):57–63. https://doi.org/10.1590/s0103-50532001000100008
Ogolo N, Olafuyi O, Onyekonwu M (2012) Enhanced oil recovery using nanoparticles. In: SPE Saudi Arabia section technical symposium and exhibition. https://doi.org/10.2118/160847-ms
Shen D, Zhang P, Kan AT, Fu G, Alsaiari HA, Tomson MB (2008) Control placement of scale inhibitors in the formation with stable Ca-DTPMP nanoparticle suspension and its transport in porous medium. In: SPE international oilfield scale conference. https://doi.org/10.2118/114063-ms
Tantayakom V, Fogler HS, Charoensirithavorn P, Chavadej S (2005) Kinetic study of scale inhibitor precipitation in squeeze treatment. Cryst Growth Des 5(1):329–335. https://doi.org/10.1021/cg049874d
Tian J, Xu J, Zhu F, Lu T, Su C, Ouyang G (2013) Application of nanomaterials in sample preparation. J Chromatogr A 1300:2–16
Acknowledgements
The authors are grateful for the financial support provided by Yayasan PETRONAS (grant number YUTP-015LC0-086) and the Petroleum Engineering Department, Universiti Teknologi PETRONAS.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Ishtiaq, U., Muhsan, A.S., Rozali, A.S. et al. Graphene oxide/carbon nanotubes nanocoating for improved scale inhibitor adsorption ability onto rock formation. J Petrol Explor Prod Technol 10, 149–157 (2020). https://doi.org/10.1007/s13202-019-0689-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13202-019-0689-7