Abstract
The aim of this paper is to quantify the effect of low-salinity (LS) water on oil recovery from sandstone cores at different temperatures and at various permeabilities, oil viscosities, and Ca2+ concentrations in the formation water. Six sandstone cores were waterflooded with high-salinity (HS) and LS water at various temperatures ranging from 25 to 90 °C. Four cores were allocated to oil recovery experiments, and the other two were dedicated to surface reactivity tests. The Swi and Sor of the cores were established, and then the cores were pre-aged for 3 days at 70 °C with oil in a closed container. We examined the effect of different ionic solutions (HS water, LS water, and double Ca2+ HS water) at different temperatures. The surface reactivity test cores were flooded with the same HS and LS brines that were used in oil recovery forced-imbibition experiments. During flooding, samples of the effluent were analyzed for pH and Ca2+. The absence of an oil phase enabled us to isolate and quantify the important water–rock reactions. Ca2+ desorption from the core that was aged in the double Ca2+ concentration was larger than that desorbed from the other core, but pH and pressure was less than the other core during surface reactivity tests. Due to dehydration at high temperatures, the desorption of Ca2+ decreased as the temperature increased. Also, as the temperature increased, the pH gradient between the HS and LS water effluents decreased. Oil recovery forced-imbibition experiments with a double Ca2+ concentration showed a small LS water effect at all temperatures, meaning that the cores became more water-wet; however, the LS water effect was much greater when the amount of Ca2+ in the HS water was decreased by half. Furthermore, as the core permeability and oil viscosity increased, our tests showed a greater positive effect from the LS water. This work attempts to isolate the separate effects and thus examines the oil recovery achieved with the most important LS waterflood factors, which are temperature, ion exchange, and pH.
Similar content being viewed by others
Introduction
The recent drop in oil prices has turned the oil industry to the cheapest improved oil recovery (IOR) techniques, such as low-salinity (LS) waterflooding. Also, the main incentives of low-salinity water flooding (LS water) are the environment-friendly EOR method, which also helps to avoid the reduction in the reservoirs’ energy. That LS water requires decreasing only its salinity and the active divalent cations Ca2+ and Mg2+ make LS waterflooding the cheapest IOR technique.
Waterflooding is extensively applied worldwide to improve oil recovery. It is documented that water chemistry significantly impacts the oil recovery factor (Morrow and Buckley 2011). In the past few years, many researchers have reported that the waterflooding recovery factor increased significantly by injecting high-salinity (HS) brine (seawater) into carbonate reservoirs (Strand et al. 2008; Shariatpanahi et al. 2010) and low-salinity brine into sandstone reservoirs (RezaeiDoust et al. 2011). Wettability is one of the major parameters that control the efficiency of waterflooding. The wettability of a rock surface is determined by the thickness and stability of the water film between the rock surface and the crude oil (Fathi et al. 2011).
The wettability alteration mechanisms during low-salinity waterflooding are even less understood than the high-salinity injection in sandstone reservoirs. Several possible proposed mechanisms include (1) multicomponent ion exchange (Lager et al. 2008a, b), (2) double-layer expansion (Ligthelm et al. 2009), (3) reduction in interfacial tension and increased pH (McGuire et al. 2005), (4) fines mobilization (Tang and Morrow 1999), (5) mineral dissolution (Aksulu et al. 2012), (6) organic material desorption from the clay surface (Austad et al. 2010), (7) salt-in effect (RezaeiDoust et al. 2009). However, the presence of clays in the porous media has proven to greatly impact production (Tang and Morrow 1999; Lager et al. 2008a, b). In addition, the chemical composition of the injected water has proved to be another controlling parameter (RezaeiDoust et al. 2009, 2011; Austad et al. 2010). The control of mineralogy and water chemistry in oil recovery highlight the importance of considering simultaneous water–rock interactions, including mineral dissolution, desorption, and ion exchange during the low-salinity waterflooding of sandstone rocks. On the other hand, increasing the oil viscosity also makes residual oil saturation higher (Aboujafar 2014). To our knowledge, no experimental studies have been conducted that consider the impact of the permeability on LS waterflooding. Mineral compositions, formation water salinity, and temperature significantly influence the wettability alteration while flooding by low-salinity water in sandstone (Aghaeifar et al. 2015). Aghaeifar et al. (2015) found that when the formation water salinity was as high as 200,000 ppm at 100 °C, no LS water effect on oil recovery was observed. However, the LS water effect was significant when the formation water salinity was reduced to 23,000 ppm. On the other hand, incremental oil recovery was ascribed to the decreasing injection water salinity. Alhuraishawy et al. (2016) examined several LS water salinities (1, 0.1, and 0.01% NaCl), which produced oil recovery of 34, 40, and 45%, respectively.
Brady et al. (2015) classify the pH during LS flooding and the effect of low-salinity waterflooding into three categories: (1) pH < 5, because the low positive charge of oil to kaolinite means that the LSW can weaken oil’s affinity toward kaolinite; (2) pH 5–6, which is the strongest LS water effect due to the ability of LS water to convert the interactional forces to cause repulsion between oil and kaolinite. (3) pH > 6, because at all salinities the repulsion forces switch to the interactions forces.
When LS water displaces HS water Ca2+ desorbed from the clay surface because an ion exchange (H+ substitutes Ca2+) at the negative sites of the clay surface (Eq. 1); Then, organic materials will be desorbed from the clay due to the reaction between the protonated basic and adsorbed acidic materials with OH− (Eqs. 2, 3) (Austad et al. 2010).
Six outcrop cores were used in this paper, four for oil recovery and two for surface reactivity tests. This work aims to answer the following questions:
-
(1)
Can the varying Ca2+ concentration in formation water affect the LS water-enhanced oil recovery (EOR) mechanism(s)?
-
(2)
Can different permeabilities affect oil recovery by LS waterflooding?
-
(3)
Would the oil recovery be the same with different oil viscosities?
-
(4)
Would the different Ca2+ concentrations in the formation water affect the desorption of Ca2+ during LS waterflooding?
-
(5)
Is temperature an important factor in relation to all the above questions?
Methodology
Materials
Three brines were prepared for all experiments (Table 1). Two kind of synthetic oil were used, the viscosities were 37 and 190 cp oil. Outcrop Berea sandstone was sampled (2.54 diameter) from 12ʺ × 12ʺ × 12ʺ core samples. The core dimensions and properties are delineated in Table 2.
Setup
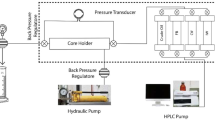
The simple model made of sandstone cores and a core holder was built to study the effect of key parameters on oil recovery, brine concentration, oil viscosity, permeability, and temperature. Schematics of the model used in these experiments are presented in Fig. 1. This model includes a core holder, an accumulator, a syringe pump, and a deionized (DI) water source.
Initial oil saturation
All cores had already been saturated with formation water (Core#1, 2, and 4 with high-salinity HS water) (Core#3 with high-salinity HS* water). All cores then flooded with 1.5 pore volume in each direction at 0.5 ml/min to displace water in order to build the irreducible water saturation (Swi) and initial oil saturation. Oil and water production were collected to calculate the original oil in place (OOIP) and Swi.
Surface reactivity test (SRT)
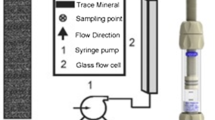
SRT were conducted according to the method described by Aksulu et al. (2012). Two cores were allocated for this test, which was conducted at three temperatures, ranging from 25 to 90 °C. The cores were evacuated and aged in HS water for 3 days at 70 °C. SRT#1 was aged in HS⃰ water, while SRT#2 was aged in HS water. The cores were then flooded with HS water at 25 °C until the pH stabilized. The fluid was then switched to LS water at 25, 70, and 90 °C until the pH stabilized. The effluent samples were collected using a fraction collector (2–3 ml) for Ca2+ analyses and pH measurements.
Ca2+ analysis
The concentrations of Ca+2 were measured by inductively coupled plasma-optical emission spectrometry using a PerkinElmer® 2000D ICP-OES. The samples were diluted in a 2% HN03 solution. QC 5PPM was run between and at the end of the samples to check the instrument performance and also to spike the sample; the spike recovery was 97%.
Results
The cores were pre-aged in oil at 80 °C for 2 weeks. In all experiments, HS water was injected into the core at 25 °C. When no more oil was produced and the stable pressure dropped across the core, the injected fluid was switched to LS water at the same temperature until no incremental oil recovery was obtained with a constant pressure drop. While the injected fluid remained the same, the temperature was increased to 70 °C until the same conditions mentioned previously were observed. Finally, the temperature was raised to 90 °C with the same LS water until a stable pressure drop occurred across the core, and no oil recovery was observed. The oil viscosity was the same for core#1, core#3, and core#4, but not for core#2. Core#2 had a higher viscosity than the others to study the effect of the oil viscosity on the oil recovery by LS waterflooding.
Effect of temperature on oil recovery by LS water, core#1
The same aging HS water of ~ 100,000 ppm (containing 89 μμCa2+) was injected into the core at 25 °C. More ultimate oil recovery was observed with increasing temperature (Fig. 2). The oil recovery from HS waterflooding was 50% OOIP. When LS water [containing zero Millimole (μmole) Ca2+] was then injected into the core at the same temperature, the oil recovery factor increased by 4.55%. When the same LS water was injected into the core at 70 °C, oil recovery increased significantly, by 5.45% OOIP. There was also an increase in the oil recovery of about 2.27% when the temperature changed to 90 °C, meaning that the LS water at all temperatures combined enhanced the oil recovery by 12.27% OOIP (Fig. 2). Figure 2 shows a normal pressure trend at 25 °C for both HS and LS water and at 70 and 90 °C for LS water. The pH was 7.14 while flooded with HS water. Upon switching to LS water, the pH rose to 8.9 at the same temperature and then began to decrease to 8.69 and 8.1, at 70 and 90 °C, respectively. The trend related to changes in the pH for the LS effluent is clearly a function of temperature (Fig. 3).
Effect of oil viscosity and temperature on oil recovery by LS water, core#2
The oil viscosity used for this core was higher than the oil viscosity in core#1. The ultimate oil recovery for this core from HS waterflooding was 48% OOIP at 25 °C. This was lower than the value for core#1, which was 50% OOIP at the same temperature. Upon switching to LS water, the ultimate oil recovery jumped by 6% OOIP at 25 °C. At 70 °C, there was no oil recovery by the same LS water, while at 90 °C, there was about 4% OOIP. The ultimate oil recovery between HS water at 25 °C and LS waterflooding at all temperatures (i.e., 25, 70, and 90 °C) was 10% compared to 12.27% for the reference core (core#1) (Fig. 4). The injection pressure increased rapidly until the Sor was established. Figure 4 shows that as the temperature increased, the pressure decreased. The pH for the HS water effluent was 7.09. The water was then switched to the LS water; the pH for the LS water effluent was ~ 9.0. Next, changing the temperature to either 70 or 90 °C stabilized the pH at 8.8 and 8.5, respectively (Fig. 5).
Effect of Ca2+ concentration and temperature on oil recovery by LS water, core#3
The concentration of Ca2+ was doubled in the aging water (HS*) for this core. Each core was aged in 89 μmole, except this core, which used 178 μmol to investigate the role of the potential determining ion toward sandstone which is Ca2+. The same HS* water was used for flooding in the oil recovery test. The ultimate oil recovery was less than that observed in core#1, which was itself lower than from core#2. Core#3 was retrieved from the same Berea sandstone. The ultimate oil recovery was about 38.2% OOIP, after the core was flooded with HS* water at 25 °C. Upon switching the injected brine to LS water, the incremental oil recovery was 1.09, 1.82%, and 0.73 at 25, 70, and 90 °C, respectively (Fig. 6). As pointed out in the Core#1, the pH jumped during LS water flooding. As the temperature increased, the LS water effluent pH tended to decrease (Fig. 7). The injection pressure proportioned inversely with temperature. However, the injected pressure did not rise as much as in the core#1 run (Fig. 6).
Effect of permeability and temperature on oil recovery by LS water, core#4
To investigate the permeability effect on LS water EOR, this outcrop core was sampled from a higher permeability than the three previous triplet cores. Sequential waterflooding was conducted on this core in the same manner as previously described. The HS water that was injected initially provided ~ 65% OOIP ultimate oil recovery, which was greater than that observed in core#1. However, the opposite was observed when LS water was injected, except at 90 °C. The ultimate oil recovery was 2.22, 0.89, and 2.23% OOIP at 25, 70, and 90 °C, respectively (Fig. 8). In addition, a lowest injected pressure was recorded in this core. Figure 9 shows an important pH jump after switching from the HS water to LS water. We observed a strong link between temperature and pH: the pH decreased as the temperature increased. The LS water injected pressure followed the same trend.
Surface reactivity tests (SRTs) Analyses
SRT1 and SRT2 core samples were taken close together and from the same zone as the triplet cores used in the oil recovery test.
SRT1 and SRT2
Figure 10a, d shows the effluent Ca2+ concentration, where SRT1 contains twice as much Ca2+ as SRT2. The concentration of Ca2+ during the HS waterflooding stabilized below 170 μmol (which was slightly below the injected Ca2+), meaning that some of the Ca2+ linked to the kaolinite negative surface. The concentration remained the same until the injected fluid was switched to LS water. The low-salinity fluid contained no Ca2+. After the LS water invaded the core, Ca2+ desorption occurred at all temperatures; however, for SRT1, desorption does not reveal a clear trend with temperature (desorption at 90 °C greater than at 70 °C), meaning that an ion exchange occurred between the LS water and sandstone. Quartz and kaolinite exchanged H+ with Ca2+. Figure 10b, e presents the increase by 1.20 pH units with LS waterflooding, while the pH rose to 2.13 in the SRT2. The pressure increase in SRT1 was not as pronounced as that in SRT2, meaning that a lower Ca2+ concentration resulted in a higher LS water efficiency (Fig. 10c, f).
Discussion
While water chemistry, temperature, oil viscosity, Ca2+ concentration, and permeability all affected the oil recovery, the Ca2+ concentration had the lowest positive impact. In contrast, temperature, oil viscosity, and permeability had the largest positive impacts. The more reservoir permeability, the more ultimate oil recovery. On the other hand, with higher Ca2+ concentrations in the formation water, there was less ultimate oil recovery. The highest impact on the pH was in core#2 (Fig. 11). The pH jump in the LS effluent for all cores at all temperatures is attributed to the ion exchanges of Ca2+ with H+ and Na+ with H+ (Austad et al. 2010; Brady et al. 2015). The alkaline environment was able to alter the wettability toward being more water-wet (Eq. 1) and (Eq. 4, Wieland et al. 1994). Figure 12 shows that as the temperature rose, measurement errors increased.
We observed a delay in the breakthrough of the early injected water between approximately 0.5–1.0 injected pore volume, at 25 °C only; after that, the oil started to flow. One explanation is after the HS water was injected into the core, adsorption of Ca2+ occurred on the negative site of the clay because HS water contains 89 μmol of Ca2+. When the LS water invaded the core, the ion exchange started. Then, a higher pH was observed in the effluent, and the pressure rose rapidly. That promoted reservoir water-wetness, which improved the microscopic sweep efficiency. The injection pressure might have increased because after the breakthrough delay and ion exchange occurred, the LS water flows deep through the pores and that could be the reason of increasing microscopic sweep efficiency. Also, as the temperature rose, the oil viscosity decreased. That would explain the lower injected pressure during the LS at 70 and 90 °C. The injected pressure in all stages for core#4 was lower than for core#1 and core#2 because the microscopic sweep efficiency in core#4 was not as pronounced as those in core#1 and core#2. The same pressure trend was observed in core#3 because more water-wetness makes the injected LS water flow through the core without obstruction (Figs. 2, 4, 6, 8).
Figure 13 displays a comparison of the ultimate oil recovery for all cores at all temperatures. For HS water at 25 °C, core#4 had the highest ultimate oil recovery due to the high permeability among the other cores, which is ascribed to the higher volumetric sweep efficiency. The highest ultimate recovery from LS water at 25 °C was for core#2, while zero ultimate oil recovery was found at 70 °C for core#2. The largest percent of oil was recovered for core#1 and core#2 at 70 and 90 °C, respectively. The most effective factors are detailed below, showing the rankings of the cores from best to worst:
-
a.
HS 25 °C: (1) core#4, (2) core#1, (3) core#2, and (4) core#3.
-
b.
LS 25 °C: (1) core#2, (2) core#1, (3) core#4, and (4) core#3.
-
c.
LS 70 °C: (1) core#1, (2) core#3, (3) core#4, and (4) core#2.
-
d.
LS 90 °C: (1) core#2, (2) core#4 ≈ core#1, and (3) core#3.
-
e.
Total LS: (1) core#1, (2) core#2, (3) core#4, and (4) core#3.
-
f.
Total recovery rankings: (1) core#4, (2) core#1, (3) core#2, and (4) core#3.
Therefore, the most effective factor influencing the ultimate oil recovery in overall flooding (HS water) was permeability, as in core#4, while temperature (only in core#1) was the most effective for LS waterflooding. Remember that core#2 was aged in high-viscosity oil, as the viscosity increased, the ultimate oil recovery increased in the LS water at 25 and 90 °C. Related to the Core#3 results, Aghaeifar et al. (2015) performed a systematic study of the relationship between the formation salinity and the LS EOR effect. When the formation water was used for aging and the flooding salinity was 200,000 ppm (640 (μmole) Ca2+ concentration), no LS EOR effect was observed because the high formation water salinity turned the initial rock wettability into strongly water-wet; however, when that water was changed to ~ 22,000 ppm (3.5 μmol Ca2+ concentration), an LS EOR effect was observed. However, when we kept the salinity at the same level (~ 100,000 ppm), but the concentration of Ca2+ was doubled (from 89 to 178 μmol), more water-wetness occurred; in turn, we found a lower LS EOR effect than for the other cores because of the capillary the capillary effect, because of that and for further investigations, the surface reactivity tests (SRTs) were performed. The Ca2+ desorption for SRT1 was larger than for SRT2, but SRT2 showed a clear trend with temperature. (As the temperature increased, the desorption decreased.) The ion exchange for SRT1 was greater than for SRT2, but the pH surge for SRT2 was larger than for SRT1. Also, the pressure was much higher for SRT2 when the LS water invaded the core, meaning that the lower the Ca2+ concentration in the formation water, the great the effect of the LS water. The increase in pressure in SRT2 indicates that LS water improved the microscopic sweep efficiency, but for SRT1, the water flowed with no obstruction. The low Ca2+ concentration in SRT2 promoted a more alkaline environment, which altered the wettability, making it more water-wet.
Conclusion
Ca2+ desorption is associated with a pH jump; thereby, an increase in the microscopic sweep efficiency was the key point of this work at a different temperatures. In addition, oil viscosity and permeability at various temperatures had a large impact on the LS water EOR. The following conclusions were established:
-
1.
The HS* water (doubled Ca2+) shifted the wettability toward strongly water-wet, despite the salinity being the same, which would obviously lower the ultimate oil recovery.
-
2.
The ultimate oil recovery was observed with varying temperatures in core#3, but the effect of temperature was not as pronounced as that of the Ca2+ concentration.
-
3.
Higher permeability promotes volumetric sweep efficiency, thereby improving the ultimate oil recovery during secondary and tertiary oil recovery.
-
4.
That the alkaline environment created after the LS water invaded the porous media induced the wettability to become more water-wet.
-
5.
High oil viscosity promoted oil recovery, especially at LS 25 °C and LS 90 °C.
-
6.
As the temperature increased, more banks of movable oil were obtained because higher temperatures reduce oil viscosity.
-
7.
Surface reactivity tests showed that ion exchange is important for oil recovery but not essential, especially when a large amount of Ca2+ exists in the formation water.
References
Aboujafar SM (2014) Effect of oil viscosity and brine salinity/viscosity on water/oil relative permeability and residual saturations. In: SPWLA 55th annual logging symposium, Abu Dhabi, 18–22 May
Aghaeifar Z, Strand S, Austad T, Puntervold T, Aksulu H, Navratil K, Storås S, Håmsø D (2015) Influence of formation water salinity/composition on the low-salinity enhanced oil recovery effect in high-temperature sandstone reservoirs. Energy Fuels 29(8):4747–4754
Aksulu H, Håmsø D, Strand S, Puntervold T, Austad T (2012) Evaluation of low-salinity enhanced oil recovery effects in sandstone: effects of the temperature and pH gradient. Energy Fuels 26:3497–3503
Alhuraishawy AK, Imqam A, Wei M, Bai B (2016) Coupling low salinity water flooding and preformed particle gel to enhance oil recovery for fractured carbonate reservoirs. In: SPE western regional meeting, Anchorage, Alaska, 23–26 May. SPE-180386-MS. http://dx.doi.org/10.2118/180386-MS
Austad T, RezaeiDoust A, Puntervold T (2010) Chemical mechanism of low salinity water flooding in sandstone reservoirs. In: proceedings of the 2010 society of petroleum engineers (SPE) improved oil recovery symposium, Tulsa, Oklahoma, 24–28 April. SPE-129767
Brady PV, Morrow NR, Fogden A, Deniz V, Loahardjo NW (2015) Electrostatics and the low salinity effect in sandstone reservoirs. Energy Fuels 29(2):666–677
Fathi J, Austad T, Strand S (2011) Water-based enhanced oil recovery (EOR) by smart water: optimal ionic composition for EOR in carbonates. Energy Fuels 25(11):5173–5179
Lager A, Webb KJ, Black CJJ, Singleton M, Sorbie KS (2008a) Low salinity oil recovery—an experimental investigation. Petrophysics 49(1):28–35
Lager A, Webb KJ, Collins IR, Richmond DM (2008) LoSal enhanced oil recovery: evidence of enhanced oil recovery at the reservoir scale. In: Proceedings of the 2008 society of petroleum engineers (SPE) symposium on improved oil recovery, Tulsa, Oklahoma, 19–23 April. SPE-113976
Ligthelm DJ, Gronsveld J, Hofman JP, Brussee NJ, Marcelis F, van der Linde HA (2009) Novel waterflooding strategy by manipulation of injection brine composition. In: EUROPEC/EAGE conference and exhibition, Amsterdam, 8–11 June. SPE-119835. http://dx.doi.org/10.2118/119835-MS
McGuire PL, Chatham JR, Paskvan FK, Sommer DM, Carini FH (2005) Low salinity oil recovery: an exciting new EOR opportunity for Alaska’s North Slope. In: SPE western regional meeting, Irvine, California, 30 March–1 April. http://dx.doi.org/10.2118/93903-MS
Morrow N, Buckley J (2011) Improved oil recovery by low-salinity waterflooding. J Pet Technol 63(05):106–112
RezaeiDoust A, Puntervold T, Strand S, Austad T (2009) Smart water as wettability modifier in carbonate and sandstone: a discussion of similarities/differences in the chemical mechanisms. Energy Fuels 23(9):4479–4485
RezaeiDoust A, Puntervold T, Austad T (2011) Chemical verification of the EOR mechanism by using low saline/smart water in sandstone. Energy Fuels 25:2151–2162
Shariatpanahi SF, Strand S, Austad T (2010) Evaluation of water-based enhanced oil recovery (EOR) by wettability alteration in a low-permeable fractured limestone oil reservoir. Energy Fuels 24:5997–6008
Strand S, Austad T, Puntervold T, Høgnesen EJ, Olsen M, Barstad SMF (2008) “Smart water” for oil recovery from fractured limestone: a preliminary study. Energy Fuels 22:3126–3133
Tang GQ, Morrow NR (1999) Influence of brine composition and fines migration on crude oil brine rock interactions and oil recovery. J Pet Sci Technol 24:99–111
Wieland E, Wanner H, Albinnson Y, Wersin P, Karnland O (1994) A surface chemical model of the Bentonite−Water interface and its implications for modelling the near field chemistry in a repository for spent fuel, SKB Technical Report 94–26. Svensk Kärnbränslehantering AB, Stockholm
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Al-Saedi, H.N., Alhuraishawy, A.K., Flori, R. et al. Sequential injection mode of high-salinity/low-salinity water in sandstone reservoirs: oil recovery and surface reactivity tests. J Petrol Explor Prod Technol 9, 261–270 (2019). https://doi.org/10.1007/s13202-018-0466-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13202-018-0466-z