Abstract
The aim of this work was to evaluate the removal of Cd (II) from water using three biosorbents originated from the biomass of jatropha (bark, endosperm, and endosperm + tegument). For that, batch tests were performed to verify the effect of solution pH, adsorbent mass, contact time, initial concentration of Cd (II), and the temperature of the process. The adsorption process was evaluated by the studies of kinetics, isotherms, and thermodynamics. The ideal conditions of solution pH were 5.5 and 8 g L−1 of adsorbent mass of biosorbents by solution volume, with an equilibrium time of 60 min. According to the Langmuir model, the maximum adsorption capacity for bark, endosperm, and bark + endosperm of jatropha was, respectively, 29.665, 19.562, and 34.674 mg g−1, predominating chemisorption in monolayers. The biosorbents presented potential for the remediation of waters contaminated with Cd (II).
Similar content being viewed by others
Introduction
Metal pollution caused by the modern anthropic activities in the industry, agricultural, and mining sectors is causing a great concern worldwide (Galunin et al. 2014). As everybody knows, toxic metals are the main pollutants of the highly relevant water resources and environment (Li et al. 2014).
Among the toxic metals, cadmium (Cd) has a very high toxic potential, and it is perhaps the seventh most dangerous chemical element in the world (ATSDR 2008). Cadmium has several uses of which the most important are batteries, pigments, plastics, and agricultural pesticides (Yavuz et al. 2007). The metal may also be frequently found as a contaminant substance in agricultural fertilizers and in wastes from zinc, copper, and lead-mining activities (ICdA 2001). Mining and casting are the main sources of Cd in water (drain water, waste water, discharge reservoir, and run-off rain water in mining areas), welding operations, application of contaminated fertilizers, treatment plants of urban, and industrial drainage, and deposits and earth fillings with dangerous residues (ATSDR 2013).
Cadmium has a high accumulative capacity in the human body and harms the cardiovascular (heart and blood vessels), gastro-intestinal (digestive), neurological (nervous system), kidney (urinary system and kidneys), reproduction, and respiratory (from the nose to the lungs) systems, coupled to devastating effects during the development of the organs. In fact, it is one of the most powerful carcinogenic agents (ATSDR 2013; Zhang et al. 2014).
Several strategies, mainly chemical and physical precipitation, ionic exchange, extraction by solvents, bio-accumulation by aquatic plants or micro-organisms, and filtration by membranes and adsorption, have been deployed to remove metal contaminants from water solutions (Li et al. 2014; Jimenez 2004; Bessbousse et al. 2008; Ali et al. 2013).
Although activated carbon is the main adsorption material, its high production costs have a negative impact on its ranking in contaminant removal on a large scale (Santhi et al. 2010). The employment of alternative materials for the production of adsorbents is increasing the object of research, where the main sources of prime matters are agro-industrial wastes (Valdman et al. 2001). It should be underscored that the use of agro-industrial wastes is advantageous due to low costs, lack of any treatment, and derivation from renewable sources.
The jatropha (Jatropha curcas L.), commonly known as Barbados nut, is an oil-producing plant for the production of biodiesel in several countries worldwide (FACT 2010). Great amounts of vegetal wastes, currently with low usage, are generated after oil extraction from the seeds (Ye et al. 2009). The possible usage of residues as an adsorbent of toxic compounds, such as Cd, may increase the value of the species’s productive chain and may de-contaminate the environment through an economical sustainability of its cultivation.
The current research evaluates the removal capacity of Cd2+ from water solutions by three biosorbents obtained from the jatropha biomass (bark, endosperm, and endosperm + peel) in different experimental conditions (adsorbent mass, solution’s pH, contact time, and initial concentration of Cd2+ in the solutions) by the adsorption process, determining kinetic, equilibrium, and thermodynamic parameters of the adsorptive process. The three evaluated adsorbents were also compared with a commercial adsorbent.
Materials and methods
Obtaining and preparing the adsorbent materials
The adsorbent materials (biosorbents) evaluated were produced from the biomass of jatropha fruit harvested from plants cultivated at the experimental farm of the Universidade Estadual do Oeste do Paraná (Unioeste) at Pato Bragado PR Brazil. Fruit harvest occurred in 4-year-old plants, and their cultivation occurred without any sort of fertilization. Harvested jatropha fruits were dried in the shade for 45 days and stored for later use.
The three adsorbent materials were produced from different types of waste of the jatropha fruit: the bark of the plant produced the first adsorbent (adsorbent C); the second adsorbent (adsorbent E) was obtained from the cake after oil extraction from the endosperm of the jatropha seeds; and the third adsorbent (adsorbent E + T) was obtained from the cake after the oil extraction of the endosperm coupled to the peel of the jatropha seeds. Although the peel of jatropha fruit is not used for biodiesel production, it was evaluated, since it is the first residue material produced in fruit processing. The chemical extraction generally used in jatropha oil extraction (extraction by solvents) produces wastes similar to adsorbent E, whereas the mechanical extraction of oil from the jatropha seeds (pressing) produces a residue similar to that used for the adsorbent E + T (FACT 2010). Consequently, the three adsorbents simulated true conditions for the re-use of the residues of the activity.
After drying, the jatropha fruits were divided into the three above-mentioned prime matters. The latter were ground and sieved in a 14–60 mesh granulometry and then dried in forced air buffer at 60 °C for 48 h. In the case of adsorbents E and E + T, vegetal oil was extracted by the Soxhlet method for 4 h after the dehydration of the prime matters (IUPAC 1988). Drying followed in a buffer at 80 °C for 24 h for the total evaporation of the solvent (n-hexane) used in the extraction of vegetal oil.
Characterization of biosorbents
The three adsorbents evaluated in the current assay were characterized by their functional groups, their morphology, by the quantification of their surface loads and the metals in their structure. The biosorbents’ functional groups were performed by infrared spectra with a Shimadzu Infrared Spectrophotometer FTIR-8300 Fourier Transform, in the region between 400 and 4000 cm−1, with 4 cm−1 resolution. Spectra were obtained by transmittance with potassium bromide pellets (KBr).
The morphology of the biosorbents was characterized by a Scanning Electron Microscope (SEM) in a microscope FEI Quanta 200 at 30 kV. Procedure comprised samples placed on a double surface carbon adhesive tape fixed on a sample support, followed by gold metallization up to a thickness of approximately 30 nm with a Baltec Scutter Coater SCD 050.
Surface loads were quantified by determining the point of zero charge (pHPCZ) of the adsorbents. In fact, pHPCZ is the pH in which the adsorbents’ equilibrium of surface loads is nil. Procedure consisted in adding, for each material, 50 mg of biosorbent mass to 50 mL of potassium chloride water solutions (KCl, Vetec, 99 %) at 0.05 and 0.5 mol L−1, with the initial pH rates ranging between 2.0 and 9.0, and adjusted to hydrochloric acid (HCl, Vetec, 37 %) or sodium hydroxide (NaOH, Vetec, 99 %) solutions at a concentration of 0.1 mol L−1. After stirring for 24 h (200 rpm) in each solution, the final pH rates were calculated. A graph on the variations of the initial pH related to the final pH was produced. The point of nil rate of pH variation corresponded to pHPCZ (Mimura et al. 2010).
A nitroperchloride digestion of the materials was performed for the initial quantification of the metals in the biosorbents structure with the posterior determination of potassium (K), calcium (Ca), magnesium (Mg), copper (Cu), iron (Fe), manganese (Mn), zinc (Zn), cadmium (Cd), lead (Pb), and chromium (Cr) concentrations by flame atomic absorption spectrometry (FAAS).
Preparation of strengthened solutions and experimental conditions
All Cd2+ solutions in the current assay were obtained by diluting Cd nitrate salt [Cd(N2O6).4H2O], purchased from Sigma-Aldrich®, grade P.A., purity higher than 99 % and diluted in ultrapure water type I.
All adsorption tests were done by triplicate in batches of 125 or 50 mL Erlenmeyer’s flasks. After each test, the solutions were filtered with quality filter paper (Unifil) to separate biosorbent and liquid solution. Aliquots of liquid solution were retrieved to determine the amount of Cd2+ by FAAS.
Effect of adsorbent mass and pH of solution on the adsorption process
Experiments with mass quantity and pH were performed to obtain the ideal quantity of adsorbent mass to be employed and the best pH condition of the solution during the adsorption process. Different mass quantities (0.2, 0.4, 0.6, 0.8, 1.0, and 1.2 g) for each biosorbent were tested in different Cd2+ solutions with four pre-defined pH conditions (4.0, 5.0, 6.0, and 7.0). Furthermore, pH rates were adjusted with HCl (Vetec, 37 %) or NaOH (Vetec, 99 %) solutions, at 0.1 mol L−1 concentration. At this stage, stirring speed (200 rpm), stirring time (1.5 h), temperature of solutions (25 °C), and Cd2+ concentration in the solutions (10 mg L−1) were constant.
After the batch tests, the solutions were filtered with quality filter paper (Unifil) to separate the biosorbent and the liquid solution. The filter paper did not influence in the results, because it was used the same kind of filter paper for all samples. The quantity of Cd2+ in the solution was determined. Cd2+ removal percentage from the solutions could be obtained from the results by Eq. (1):
where %R is the removal percentage of the metal by the biosorbent; C eq is the concentration of Cd2+ in equilibrium (mg L−1); and C 0 is the initial concentration of Cd2+ in the solution (mg L−1).
All the experiments were conducted in laboratory and in controlled environment, and concentrations were determined by FAAS using the Cd certified standards.
Effect of contact time between adsorbent and adsorbed and the kinetics of adsorption
After defining mass quantity (0.4 g) and ideal pH (5.5), tests evaluated the effect of contact time between adsorbent and adsorbed (5, 10, 20, 40, 60, 80, 100, 120, 140, and 160 min). Stirring speed (200 rpm), temperature of solutions (25 °C), and Cd2+ concentrations in the solutions (10 mg L−1) were kept constant, whilst the solutions’ pH was adjusted with HCl (Vetec, 37 %) or NaOH (Vetec, 99 %) solutions at 0.1 mol L−1. Amount adsorbed in the equilibrium gave rise to the rate obtained for the concentration in equilibrium by Eq. (2):
where Q eq is the quantity of Cd2+ adsorbed per unit of adsorbent in equilibrium (mg g−1); m is the biosorbent’s mass used (g); C 0 is the initial concentration of Cd2+ in the solution (mg L−1); C eq is the Cd2+ concentration in the solution in equilibrium (mg L−1); and V is the volume of the solution employed (L).
Results were employed to explain the kinetic mechanism that controls the adsorption process by means of the pseudo-first-order, pseudo-second-order, Elovich, and intraparticle diffusion models.
Effect of the initial concentration of the adsorbate in the adsorptive process
The analysis of the effect of different initial concentrations of Cd2+ in solution (5, 20, 40, 60, 80, 100, 120, 140, 160, and 200 mg L−1) was performed with ideal conditions of the biosorbents’ mass (0.4 g), pH of solution (5.5), and contact time (60 min). The solutions’ stirring speed (200 rpm) and temperature (25 °C) were constant during the procedure. Furthermore, pH of the solutions was adjusted with HCl (Vetec, 37 %) or NaOH (Vetec, 99 %) solutions at 0.1 mol L−1 concentration.
Equations (1) and (2), respectively, provided the removal percentage of Cd2+ (%R) and the amount adsorbed in equilibrium (Q eq). The latter parameter is used to obtain adsorption isotherms which were linearized by the mathematical models of Langmuir, Freundlich, and Dubinin–Raduschkevich (D–R).
In the current assay, the comparison of Cd2+ removal capacity between jatrophas’s adsorbents and a commercial adsorbent was undertaken by experiments within the same conditions for biosorbents, or rather, activated coal P.A. Synth®, widely used in the removal of pollutants in water matrixes (Oliveira et al. 2008).
Another procedure consisted in determining the structural components in the biosorbents after the adsorptive process. Samples of biosorbents were removed after adsorption process and submitted to IR spectrometric techniques within the same conditions, as described in “Characterization of biosorbents”.
Desorption assay
Tests were performed with masses obtained after experiments involving different initial concentrations of Cd2+ to evaluate the Cd2+ desorption of biosorbents compared with commercial adsorbent (activated coal). Masses were obtained by filtering solutions in equilibrium coupled to drying in buffer at 60 °C for 24 h. Each biosorbent mass was then placed in contact with 50 mL of HCl solution (Vetec, 37 %) at 0.1 mol L−1, followed by the stirring of the solutions at 200 rpm, at 25 °C for 60 min. After determining the concentration of Cd2+ desorbed in solution, the desorption percentage was calculated by Eq. (3):
where %D is the percentage of Cd2+ desorption by the biosorbent; and C eq(des) (mg L−1) and C eq(ads) (mg L−1) are, respectively, the concentrations of desorbed and adsorbed Cd2+ in equilibrium by the biosorbent.
Effect of temperature in the adsorption process (thermodynamics)
Tests at different solution temperatures (15, 30, 45, and 60 °C) were performed with the Cd2+ initial concentration the highest removal percentages for the three biosorbents (80 mg L−1 for biosorbent C, 120 mg L−1 for biosorbent E, and 80 mg L−1 for biosorbent E + T). During this stage, the solutions’ pH (5.5), biosorbent mass (0.4 g), stirring speed (200 rpm), and contact time between biosorbent and solution (60 min) were kept constant.
Data were used to determine thermodynamic parameters and to investigate the adsorption process. Gibbs’s free energy (ΔG) (Eq. 4), enthalpy (ΔH), and entropy (ΔS) (Eq. 5) were calculated (Sari et al. 2007; Gonçalves et al. 2008):
where K d is the ratio Q eq/C eq; R is the universal constant of gases (8.314 J mol−1 K−1); T is the temperature (K) used in adsorption tests; and ΔH and ΔS rates were obtained by lnK d as a function of 1/T.
Results and discussion
Characterization of biosorbents
Figure 1 shows the IR spectra of the three biosorbents under the analysis in the current assay, with vibrations at bands 760, 1062, 1370, 1542, 1650, 2900, and 3400 cm−1.
The wide and strong band in 3400 cm−1 for the three biosorbents may be attributed to the vibration lengthening of the hydroxyl bonds (O–H) usually found in carbohydrates, fatty acids, proteins, lignin units, cellulose, and absorbed water (Stuart 2004; Garg et al. 2008; Gonçalves et al. 2010; Han et al. 2010; Feng et al. 2011). Vibration lengthening in 2900 cm−1 may be related to C–H bonds in alkane groups and aliphatic acids (Barbosa 2007; Iqbal et al. 2009). Bands in 1650, 1542, and 1370 cm−1 may be attributed to the vibration lengthening of C–O-type bonds found in starch and carboxyl groups (Han et al. 2010). Moreover, vibration in 1650 cm−1 may also be provided by C = O-type bonds, which constitutes carbonyl and carboxylate groups in lignocellulose compounds (Jacques et al. 2007; Tongpoothorn et al. 2011). Bands in 1542 and 1370 cm−1 may also occur due to C = C bonds of aromatic compounds (Pavan et al. 2008). It should be underscored that 1370 cm−1 band may be provided by the vibration of C–H-type bonds of celluloses, hemicelluloses, and lignin (Smith 1999). Vibration lengthening in 1062 cm−1 may be related to C–O-type bonds in lignin and in phenols, which contains carboxyl groups that make available adsorption sites for metallic ions (Pascoal-Neto et al. 1995; Guo et al. 2008; Barka et al. 2010). Band in 760 cm−1 for biosorbent from jatropha peel shows the probable presence of nitrogen (N) with bio-bondings (Barka et al. 2010).
Proteins, lignin, cellulose, hemicellulose, lignocelluloses and the hydroxyl, carboxyl, and carbonyl and phenol groups are the main compounds capable of forming bonds with metallic ions in the structure of biosorbents obtained from jatropha biomass (Kurniawan et al. 2006; Demirbas 2008). As a rule, vegetal-derived materials are mainly constituted by cellulose, hemicellulose, and lignin (Pehlivan et al. 2009). It should be underscored that the results in IR spectra for the three biosorbents are similar to those reported by Tongpoothorn et al. (2011) and Prola (2013) who also experimented with the biomass of jatropha fruit biomass for the production of biosorbents.
SEM-produced evaluation of adsorbents’ morphology (Fig. 2) showed that the three materials had a highly irregular morphology which indicated a porous surface. Materials with such characteristics generally have a high specific surface, which provides good adsorption capacity.
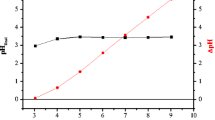
Results obtained from the determination of the biosorbents’ surface loads (pHPCZ) in the two KCl concentrations (0.05 and 0.5 mol L−1) revealed that the load equilibrium is nil when pH rates lay between 5 and 6 (Fig. 3). Or rather, equilibrium between positive and negative loads is zero in such pH conditions. When the solution’s pH is lower than pHPCZ, positive surface loads predominate on the biosorbents’ surface due to the protonation of the latter. On the other hand, when pH rates of solutions are higher than pHPCZ, the predominant surface loads are negative and cation adsorption occurs (Mimura et al. 2010). Furthermore, pHPCZ rates for the three biosorbents are close to those reported by Namasivayam et al. (2007), with pHPCZ equal 7.2 for the jatropha peel.
The quantification of metal concentrations in biosorbents prior to deployment (Table 1) showed that Cd2+ concentrations were lower than the quantification limit of the method used (FAAS). In fact, the above results were expected, since plants rarely accumulated toxic substances in their reproductive structures (Shtangeeva et al. 2011).
Effect of adsorbent mass and pH of the solution in the adsorption process
Evaluation of pH influence on the adsorption process (Fig. 4) demonstrated that Cd2+ adsorption capacity by biosorbents jatropha bark (C) and jatropha endosperm + peel (E + T) was only slightly affected by the variations of this factor. In the case of the jatropha endosperm (E), a greater Cd2+ removal occurred when the solution’s pH lay between 4.0 and 5.0.
Moreover, the pH affects the qualities of the adsorbents, functional groups, and the ionic state of the metal species. In fact, it is an important factor to be taken into account in metal ions adsorption (Hossain et al. 2012). As a rule, a strong competition occurs in acid pH by the active sites of the adsorbent between the excess of H+ ions and the metal ions in the solution. On the other hand, the adsorption’s bond sites may not be activated with an alkaline pH. There is the possibility of the complexation and precipitation of the metals in the solution, with less contact between the adsorbent and the adsorbed and, consequently, a low adsorption (Sari et al. 2007; Demirbas 2008). The co-relationship between results in the determination of pHPCZ and pH tests revealed 5.5 as the best rate for the adsorption process.
Results on the influence of mass quantity of biosorbents (Fig. 4) show that a high Cd2+ removal rate occurs as from 0.2 g, and remains relatively stable up to 1.2 g. Since this experimental stage was performed at a low Cd2+ concentration in the solution (10 mg L−1), the best mass quantity was 0.4 g, which warranted an effective removal of the metal in the subsequent stages. Since studies on the biosorbent mass were performed in 50 mL solutions, the proportion of mass per volume in L was calculated, in which 0.4 g in 50 mL corresponded to 8.0 g L−1.
Since mass variation in the adsorption process modifies the surface area of the adsorbent material and the number of active sites available for adsorption (Ofomaja 2010; Vaghetti et al. 2008) increase in mass causes an increase in these two characteristics. However, when a very high mass quantity is employed, a decrease in adsorbate removal may occur, since there is a reduction of the total surface area of the biosorbent due to the formation of aggregates and the occurrence of empty active sites within the aggregates caused by their reduced of contact with the metal ions in the solution (Montanher et al. 2005; Ho and Ofomaja 2006; Kumar and Porkodi 2007).
Average Cd2+ removal rates by biosorbents under the analysis were, respectively, 86, 74, and 82 % for the jatropha bark (C), endosperm (E), and endosperm + peel (E + T).
Study on the effect of contact time between adsorbent and adsorbate and adsorption kinetics
An analysis of contact time between adsorbent and adsorbed explains the minimum period required for equilibrium within the adsorption system. The current assay shows that the results for this factor (Fig. 5) demonstrate that after a 60-min contact, the system commences a dynamic equilibrium, with no great variation in the absorbed quantity (Q eq) after this interval. The best contact time between biosorbents and Cd2+ solution is 60 min. The above corroborates results by Garg et al. (2008) when they studied Cd2+ adsorption in jatropha cake.
The required 60 min to obtain maximum Cd2 + removal may be a short time. Longer contact periods are usually needed for high removal rates which may reach 180 min (Guo et al. 2008; Argun and Dursun 2008; Witek-Krowiak et al. 2011; Coelho et al. 2014; Meneghel et al. 2013a; Rubio et al. 2013a). It should be underscored that a quick and effective removal of solution contaminants is highly important and desirable for biosorbents. In fact, it is an asset for their viability and potential in de-contamination processes in batch or continuous flow systems.
Studies on contact time between adsorbent and adsorbed clarify the mechanisms that control the adsorption process and determined by the adsorption kinetics. A study on the adsorption kinetics by well-known and well-established models, namely, the pseudo-first, pseudo-second, Elovich, and intraparticle models. Figure 6 and Tables 2 and 3 show the application results of the models.
Linearization of Cd2+ adsorption kinetics by the biosorbents jatropha bark (C), endosperm (E), and endosperm + peel (E + T) for the pseudo-first-order (a), pseudo-second-order (b), Elovich (C), and intraparticle diffusion (d) models (t 5–180 min, C 0 10 mg L−1, m 8 g L−1, pH 5.5, 200 rpm, and 25 °C)
Studies on the diffusion models deal with the rate of the coefficient of determination (R 2) for the acceptance or rejection of the model, or rather, the closer the model is to 1.00, the better the model adjusts itself. In the case of the pseudo-first- and pseudo-second-order models, Q eq rates obtained by the models should be compared with the experimental Q eq rates and the average of Q eq rates in the contact time test (Febrianto et al. 2009). The experimental Q eq rates for biosorbents bark (C), endosperm (E), and endosperm + peel (E + T) of the jatropha were, respectively, 0.998, 1.074, and 0.927 mg g−1 (Table 2).
When the parameters given by the kinetic model of the pseudo-first order (Table 2) are evaluated, it may be seen that the model does not adjust itself adequately for the three biosorbents (R 2 rates are low and calculated Q eq rates were not close to those of the experimental Q eq rates). Consequently, there is no predominance of physiosorption in the adsorption process, since the pseudo-first-order model indicates this kind of adsorption.
According to Farooq et al. (2011), the pseudo-first-order model does not usually provide adjustment for most biosorption systems. The above has been reported in other research works that evaluated Cd2+ removal capacity from water solutions by biosorbents (Coelho et al. 2014; Meneghel et al. 2013a; Rubio et al. 2013a).
The pseudo-second-order model had the best adjustment among the kinetic models for the three biosorbents. In fact, all R 2 rates were excellent (1.000 for C and E and 0.999 for E + T), whereas the rates of calculated Q eq (1.033 mg g−1 for C, 1.106 mg g−1 for E, and 0.965 mg g−1 for E + T) were close to those by the experimental Q eq rates (0.998 mg g−1 for C, 1.074 mg g−1 for E, and 0.927 mg g−1 for E + T) (Table 2). The adsorption process among Cd2+ ions and the actives sites of the three biosorbents occurred by chemical adsorption through valence and electron sharing (Oliveira et al. 2008; Feng et al. 2011; Ho and Mckay 1999).
Since R 2 results for the Elovich model (Table 2) were very low, the kinetic model proved to be non-adjustable and, consequently, could not be used for adsorption kinetics. As a rule, the Elovich kinetic model had better adjustments in systems that involve the chemosorption of gases in solid surfaces without the products’ desorption (Tseng et al. 2003). However, several researchers are employing the model to explain the adsorption process of metal species in biosorbents, since their parameters (A and B) may be used to interpret and understand the initial rate of the adsorption process and the sites involved in the adsorption process (Hossain et al. 2012).
Intraparticle diffusion is another model for the explication of adsorption kinetics. It is largely applied for adsorbents with porous morphology and large specific surface. The evaluation of the model employs R 2 rates and C i rate which shows whether the straight line generated by the application of the model passes or not on the origin of axes of Q eq vs t 1/2. In this case, the straight line passes on the origin axes when C i rate is equal to zero. The above shows that the intraparticle diffusion is the bordering stage of the adsorption process (Rocha 2012).
Results from the application of the intraparticle diffusion model (Table 3) show that linearization for biosorbent C provided two straight lines, with relatively satisfactory R 2 rates, whereas a single straight line was generated for biosorbents E and E + T. Since the three materials had C i rates different from zero, intraparticle diffusion is not the limiting stage of the adsorption process, which may be monitored by diffusion and by external transport with surface adsorption (Vadivelan and Kumar 2005; Gundogdu et al. 2009). Ci rate also indicates the thickness of the limit layer of adsorption. Consequently, the higher its rate, the greater is the amplitude of the surface diffusion in the limiting stage of the process, especially biosorbent E in this particular case (Melo 2009).
When the applicability of all the kinetic models employed in the current research is taken into account, it may be perceived that chemosorption occurs in the adsorption process between Cd2+ ions and the three biosorbents. An exchange of electrons effectively occurs between the adsorbent and the adsorbed in such an adsorption, with a strong bond between the two components, mainly with the occurrence of polar covalent bonds (Melo 2009). Chemical adsorption with big bond forces between the adsorbate and the active sites of the adsorbent is a relevant characteristic, since it is desirable that in the de-contamination of water, the contaminants are strongly adhered to the adsorbent and do not return easily to the solution.
Effect of the initial concentration of the adsorbate in the adsorption process
Removal percentages
It should be underscored that in the evaluation of Cd2+ removal percentages by biosorbents (Table 4), the highest removal rates of the metal by the three biosorbents were obtained within the concentration interval between 60 and 100 mg L−1. Among the three biosorbent materials, the jatropha endosperm (E) had the lowest removal rates, with approximately 70 % of Cd2+ in the solution at a concentration of 200 mg L−1. On the other hand, bark (C) and endosperm + peel (E + T) had a removal capacity of approximately 90 % of the metal at the above-mentioned concentration.
In spite of the differences in removal percentages, the three materials had a similar behavior with an increase in Cd2+ concentrations, with an initial increase of removal rate and later a decrease of the parameter. A decrease in the removal of adsorbates due to an increase of the initial concentration is due to a greater occupation of the adsorption sites in the adsorbent material. The competition for these active sites may even increase, followed by a decrease in the adsorption efficiency (Puranik and Paknikar 1999; Singha and Das 2011). An increase in the concentration of the adsorbate does not provide a higher removal rate due to the saturation of active sites and a greater competition for the sites. There is consequently a lower removal rate when compared to the initial concentration.
The comparison between the removal percentages of the three biosorbents and the removal percentage of a commercial adsorbent (activated coal) shows that the latter has a Cd2+ removal capacity which is higher than adsorbents, especially with regard to endosperm + peel (E + T), as from 160 mg L−1, higher than activated coal. Removal efficiency of metallic contaminants in liquid solutions by activated coal is very well known, since it is actually a high capacity adsorbent. Comparison with this material is relevant to show the efficiency of the biosorbents under the analysis. Or rather, the biosorbents analyzed are capable of removing Cd2+ from water solutions.
It should be underscored that biosorbent materials from jatropha biomass did not undergo any modification or chemical activation, whereas activated carbon was obtained by these processes which is the main cause for its high cost (Girods et al. 2009). The use of waste materials with a good removal capacity of contaminants without modification or activation is highly desirable, since there is a decrease in the main impairments of the process, or rather, effluent treatment costs or contaminated resources.
Results as removal percentages of the metal Cd2+ from contaminated solutions were employed to obtain the amount of metal in the solutions after the adsorption process. Rates were then compared with those listed by Brazilian law resolution of CONAMA n. 357/2005 and n. 430/2011 and Ordinance n. 2914/2011 of the Ministry of Health (Brasil 2005, 2011a, b), respectively, 0.01, 0.20, and 0.005 mg L−1 of Cd2+. The lowest Cd2+ concentrations in the solutions after the adsorption process with regard to bark, endosperm, and endosperm + peel were, respectively, 0.71, 1.68, and 1.29 L−1. According to the Brazilian legislation, the solution was still contaminated. It should be emphasized that the initial concentrations of Cd2+ were high and that the contaminated solution was processed only once. Consequently, further adsorption could have de-contaminated the solutions.
Adsorption isotherms
Adsorption isotherms are the relationship between the amount of adsorbed ions per adsorbent mass and the concentration of the solution in dynamic equilibrium (Witek-Krowiak et al. 2011). The coefficients obtained in linearization are highly relevant for the interpretation of data derived from the adsorption process.
The three biosorbents under the analysis and activated carbon provided the isotherms of group L of Langmuir (Fig. 7), with the curve turned downwards due to a decrease in the availability of active sites when the adsorbate concentration increased (Witek-Krowiak et al. 2011). The above isotherm also provided maximum adsorption capacity by mathematical models, such as that by Langmuir (Montanher et al. 2005).
Isotherms obtained in the experiments with increases in the initial Cd2+ concentrations were linearized by the Langmuir, Freundlich, and Dubinin–Radushkevich (D–R) mathematical models to determine the adsorption capacity of the biosorbents and the main parameters that involve the adsorption process (Fig. 8). With regard to linearization, the applicability of the mathematical model is evaluated by the rate of the coefficient of determination (R 2). Consequently, linearization results (Table 5) show that Langmuir’s model had the best adjustment in the case of the three biosorbents and suggested that Cd2+ adsorption by these materials occurred predominantly in monolayers (Montanher et al. 2005). On the other hand, activated coal had the highest R 2 rate by Freundlich’s adjustment model (0.991). However, Langmuir’s model also provided a satisfactory R 2 rate (0.984).
When Q m rates, or rather, the highest adsorption capacity by Langmuir’s model, are evaluated, the biosorbents from the jatropha bark (C) and endosperm + peel (E + T) showed higher rates than those by activated carbon (C = 29.665, E + T = 34.674, and AC = 22.692 mg g−1). Contrastingly, jatropha endosperm (E) had the lowest adsorption capacity when compared to the three materials (E = 19.562 mg g−1), albeit very close to activated carbon. Results also showed that the adsorbent materials under the analysis had a high potential for Cd2+ adsorption. Since adsorption process conditions were optimized to evaluate the biosorbents, activated carbon could have a higher maximum adsorption under different conditions.
Rates for Langmuir’s parameter R L for the three biosorbents and activated carbon showed that the adsorption process was positive, since they lay between intervals 0 and 1 (Lin and Juang 2002).
Although Freundlich model’s R 2 rates are lower than those by Langmuir’s model, they are relatively close to 1.0, which show that the adsorption process may occur in multilayers. Consequently, the biosorbents evaluated the present adsorption in mono- and in multilayers, with more than one adsorption site type interacting with the adsorbate (Montanher et al. 2005; Gonçalves et al. 2012).
When Freundlich’s parameter n (reactivity of the adsorbent’s active sites) are higher than 1, highly energetic sites exist in the material and they are the first to be occupied by the metals (Sodré et al. 2001). All three biosorbents’ n rates are lower than 1, or rather, highly energetic sites are not extant. The above corroborates the best adjustment of Langmuir’s model which shows the existence of equal energy active sites in all the material.
In the case of Dubinin–Radushkevich’s (D–R) mathematic model, R 2 rates may be taken as satisfactory and permit the use of the parameters associated with the model to explain the Cd2+ adsorption process by the three biosorbents and by activated coal.
Sorption average energy of (E), free energy involved in the transference of 1 mol of the solute of the solution for the adsorbent surface, defines whether adsorption is physical or chemical (Wan Ngah and Hanafiah 2008). In fact, E rates between 1.0 and 8.0 kJ mol−1 indicate a physical adsorption, whereas rates higher than 8.0 kJ mol−1 indicate a chemical adsorption process (Romero-González 2005; Fávere et al. 2010). Consequently, biosorbents from jatropha endosperm (E) and from endosperm + peel (E + T) and activated coal have E rates higher than 8.0 kJ mol−1, and thus the predominance of chemical adsorption in the adsorption process. Once more the above corroborates the best adjustment of Langmuir’s model, since chemical adsorption occurs in monolayers (Montanher et al. 2005). Since E rate equivalent to 7.603 kJ mol−1 was obtained for the jatropha bark (C), physical adsorption was predominant. However, it should be underscored that the rate was close to 8.0 kJ mol−1 and that the D–R model had the lowest R 2 rate for the biosorbent. Consequently, there is no absolute certainty that the Cd2+ adsorption process in jatropha active was a physical adsorption.
When maximum adsorption capacity (Langmuir’s Q m) of the biosorbents under the analysis is compared with other biosorbents (Table 6), the materials of the current assay had the best adsorption capacities, and only lower than jatropha cake analyzed by Garg et al. (2008). In fact, they prove to be excellent materials for the removal of Cd2+ in solutions.
Characterization of adsorbent’s functional groups after the adsorption process
So that the functional groups in the structure of the biosorbents after the Cd2+ adsorption process could be characterized, their determination was performed by IR spectrometry. Spectra (Fig. 8) showed that functional groups in the characterization of non-saturated biosorbents with Cd2+ were not modified. Or rather, the biosorbents kept their former characteristics, with the participation of the functional groups mentioned in “Characterization of biosorbents” in the Cd2+ adsorption process.
Desorption
The evaluation of desorption of the contaminating material removed by the adsorbent is a relevant characteristic to evaluate the adsorbent’s renewal capacity for use in sorption/desorption cycles. A good desorption is desirable for the adsorbent due to a better use of the material and low costs in large-scale remediation processes.
Results of Cd2+ desorption of the biosorbents and activated coal (Table 7) revealed that the biosorbents from jatropha endosperm (E) and endosperm + peel (E + T) had low desorption rates (E = 89.31 and E + T = 85.79 %), whereas the jatropha bark (C) had the lowest desorption percentage (59.96 %). Activated coal had a desorption rate of 71.01 %. The above rates register that biosorbent materials have a good desorption rate that provides their recuperation in sorption/desorption systems and Cd2+ recuperation after the adsorption process.
Thermodynamics of adsorption
The study on temperature effect in adsorption is an important stage. Temperature changes the adsorption process and acts on its spontaneity and on the forces that interact in the adsorption of the solute in the adsorbent’s active sites. The thermodynamics of the adsorption process was performed to evaluate the temperature’s influence.
The current assay shows the results of the adsorption process’s thermodynamics between the biosorbents and Cd2+ ions in the solution (Table 8). Q eq rates (quantity of Cd2+ adsorbed by biosorbent mass) increase proportionally to any rise in the temperature of the solution and show that temperature interferes on the biosorption process.
As a rule, a specific literature reports that a temperature increase causes a decrease in adsorption within liquid/solid systems, since higher temperature rates increase solubility and consequently a decrease in chemical potential, with less adsorption (Khattri and Singh 2000). Nevertheless, several assays on the use of biosorbents in metal removal demonstrate that temperature rise may cause a desorption increase (Coelho et al. 2014; Meneghel et al. 2013a, b; Rubio et al. 2013b, c).
Negative enthalpy results (ΔH) from the three biosorbents suggest that the adsorption process is exothermal, or rather, the same occurs with energy release (Wan Ngah and Fatinathan 2010).
Other relevant thermodynamic parameter is the variation of Gibbs’s free energy (ΔG) which is the main criterion to indicate the spontaneity of a system with constant conditions of temperature and pressure (Crini and Badot 2008). Negative ΔG rates indicate the spontaneous type of the reaction (Wan Ngah and Hanafiah 2008), or rather, the more negative is the ΔG rate, the more energetically favorable is the adsorption process (Debrassi et al. 2011). ΔG results by the three biosorbents were negative at all temperatures, with more negative rates as temperature increased. Biosorbent obtained from jatropha endosperm + peel (E + T) had the most negative results, followed by biosorbent C, which, in its turn, had more negative results than C. Results showed that temperature increase caused an increase in the quantity of adsorbed Cd2+ due to a rise in the spontaneity of the adsorption process (Farooq et al. 2011).
Since entropy (ΔS) rates for the three biosorbents were positive, disorder, and the randomness of the solid/solution interface increased during the adsorption process and thus a higher adsorption capacity (Wan Ngah and Fatinathan 2010).
Adsorbents after the adsorption process
One of the main aspects involved in the evaluation of the viability and efficiency of a biosorbent is the possibility of recovering adsorbent material and the metal adsorbed, especially when the metal has high commercial value. The two main strategies for the recovery of the adsorbent and the adsorbate in contaminant removal systems are the regeneration process of the adsorbent (desorption) and incineration which is feasible when the materials employed in the adsorption process are highly available and low cost (Park et al. 2010).
Desorption results in the current assay revealed that biosorbents obtained from jatropha biomass might undergo the regeneration process for further uses which was a desirable characteristic in the current case. Furthermore, according to Coelho et al. (2014), agro-industrial materials may be used in the composition of cement material due to great amounts of silica in materials of organic origin.
Conclusion
Studies on factors that interfere in the adsorption process between biosorbents and Cd2+ ions showed that the best conditions comprise solution’s pH at 5.5, 8.0 g L−1 of biosorbent in the solution, and contact time 60 min.
According to adsorption kinetics results, the three biosorbents adsorbed Cd2+ ions by chemosorption, at the best adjustment of the pseudo-second-order model.
Langmuir’s mathematical model was the best that adjusted itself in the study on isotherms with regard to the three biosorbents and thus the predominance of chemosorption by monolayers. Maximum adsorption capacities by Langmuir’s model were 29.665, 19.562, 34.674, and 22,693 mg g−1, respectively, for jatropha bark (C), endosperm (E), endosperm + peel (E + T), and activated carbon (CA).
The three biosorbents provided a desorption rate that makes recuperation possible after the adsorption process, and thus, the use in sorption/desorption flows.
Thermodynamic results showed that the Cd2+ adsorption process by biosorbents is an exothermal process with an increase in the system’s disorder due to adsorption.
Based on results provided by the current assay, the three biosorbents obtained from the jatropha biomass may remove Cd2+ from water solutions and may be used as an alternative to usually employed adsorbents in remediation systems of water resources contaminated by the metal.
References
Ali H, Khan E, Sajad MA (2013) Phytoremediation of heavy metals—concepts and applications. Chemosphere 91:869–881. doi:10.1016/j.chemosphere.2013.01.075
Argun ME, Dursun S (2008) A new approach to modification of natural adsorbent for heavy metal adsorption. Bioresour Technol 99:2516–2527. doi:10.1016/j.biortech.2007.04.037
ATSDR—Agency for Toxic Substances and Disease Registry (2008) Draft toxicological profile for cadmium, 2008. http://www.atsdr.cdc.gov/toxprofiles/tp5.pdf. Accessed 15 Oct 2013
ATSDR—Agency for Toxic Substances and Disease Registry (2013) Toxic substances index. http://www.atsdr.cdc.gov/az/a.html. Accessed 15 Oct 2013
Barbosa LCA (2007) Infrared spectroscopy in the characterization of organic compounds. UFV, Viçosa, p 189
Barka N, Abdennouri M, Boussaoud A, Makhfoukm M (2010) Biosorption characteristics of cadmium(II) onto Scolymus hispanicus L. as low-cost natural biosorbent. Desalination 258:66–71. doi:10.1016/j.desal.2010.03.046
Bessbousse H, Rhlalou T, Verchère JF, Lebrun L (2008) Removal of heavy metals ions from aqueous solutions by filtration with a novel complexing membrane containing poly(ethyleneimine) in a poly(vinyl alcohol) matrix. J Membr Sci 307:249–259. doi:10.1016/j.memsci.2007.09.027
Brasil (2005) Ministério do Meio Ambiente. Resolução nº 357, Brasília, DF, p 23
Brasil (2011a) Ministério do Meio Ambiente. Resolução nº 430, Brasília, DF, p 8
Brasil (2011b). Ministério da Saúde. Portaria nº 2.194, Brasília, DF, p 33
Coelho GF, Gonçalves AC Jr, Tarley CRT, Casarin J, Nacke H, Francziskowski MA (2014) Removal of metal ions Cd (II), Pb(II), and Cr(III) from water by the cashew nut shell Anacardium occidentale L. Ecol Eng 73(1):514–525. doi:10.1016/j.ecoleng.2014.09.103
Crini G, Badot PM (2008) Application of chitosan, a natural aminopolysaccharide, for dye removal from aqueous solutions by adsorption processes using batch studies: a review of recent literature. Prog Polym Sci 33(4):399–447. doi:10.1016/j.1016/j.progpolymsci.2007.11.001
Debrassi A, Largura MCT, Rodrigues CA (2011) Adsorção do corante vermelho congo por derivados da o-carboximetilquitosana hidrofobicamente modificados. Quím Nova 34(5):764–770. doi:10.1590/S0100-40422011000500007
Demirbas A (2008) Heavy metals adsorption onto agro-based waste materials: a review. J Hazard Mater 157:220–229. doi:10.1016/j.jhazmat.2008.01.024
FACT (2010) The jatropha handbook: from cultivation to application. FACT, Holanda
Farooq U, Khan MA, Athar M, Kozinski JA (2011) Effect of modification of environmentally friendly biosorbent wheat (Triticum aestivum) on the biosorptive removal of cadmium (II) ions from aqueous solution. Chem Eng J 171:400–410. doi:10.1016/j.cej.2011.03.094
Fávere VT, Riella HG, Rosa S (2010) Cloreto de n-(2-hidroxil) propil-3-trimetil amônio quitosana como adsorvente de corantes reativos em solução aquosa. Quím Nova 33(7):1476–1481
Febrianto J, Kosasih AN, Sunarso J, Ju Y, Indraswat N, Ismadji S (2009) Equilibrium and kinetic studies in adsorption of heavy metals using biosorbent: a summary of recent studies. J Hazard Mater 162:616–645. doi:10.1016/j.jhazmat.2008.06.042
Feng N, Guo X, Liang S, Zhu Y, Liu J (2011) Biosorption of heavy metals from aqueous solutions by chemically modified orange peel. J Hazard Mater 185:49–54. doi:10.1016/j.jhazmat.2010.08.114
Galunin E, Ferreti EJ, Zapelini I, Vieira I, Tarley CRT (2014) Cadmium mobility in sediments and soils from a coal mining area on Tibagi river watershed: environmental risk assessment. J Hazard Mater 2265:280–287. doi:10.1016/j.jhazmat.2013.11.010
Garg U, Kaur MP, Jawa GK, Sud D, Garg VK (2008) Removal of cadmium (II) from aqueous solutions by adsorption on agricultural waste biomass. J Hazard Mater 154(1–3):1149–1157. doi:10.1016/j.jhazmat.2007.11.040
Girods P, Dufour A, Fierro V, Rogaume Y, Rogaume C, Zoulalian A, Celzard A (2009) Activated carbons prepared from wood particleboard wastes: characterization and phenol adsorption capacities. J Hazard Mater 166:491–501. doi:10.1016/j.jhazmat.2008.11.047
Gonçalves M, Oliveira LCA, Guerreiro MC (2008) Magnetic niobia as adsorbent of organic contaminants in aqueous medium: effect of temperature and pH. Quim Nova 31:518–522. doi:10.1590/s0100-40422008000300010
Gonçalves AC Jr, Nacke H, Fávere VT, Gomes GD (2010) Comparison between an anionic exchanger of chitosan quaternary ammonium salt and a commercial exchanger in the extraction of available phosphorus in soils. Quim Nova 33:1047–1052. doi:10.1590/S0100-4042201000050008
Gonçalves AC Jr, Strey L, Lindino CA, Nacke H, Schwantes D, Seidel EP (2012) Aplicabilidade da casca de pinus (Pinus elliottii) para adsorção de metais pesados tóxicos de soluções contaminadas. Acta Sci Technol 34(1):79–87. doi:10.4025/actascitechnol.v34i1.9585
Gundogdu A, Ozdes D, Duran C, Bulut VN, Soylak M, Senturk HB (2009) Biosorption of Pb(II) ions from aqueous solution by pine bark (Pinus brutia Ten.). Chem Eng J 153:62–69. doi:10.1016/j.cej.2009.06.017
Guo X, Zhang S, Shan X (2008) Adsorption of metal ions on lignin. J Hazard Mater 151:134–142. doi:10.1016/j.jhazmat.2007.05.065
Han R, Zhang L, Song C, Zhang M, Zhu H, Zhang L (2010) Characterization of modified wheat straw, kinetic and equilibrium study about copper ion and methylene blue adsorption in batch mode. Carbohydr Polym 79:1140–1149. doi:10.1016/j.carbpol.2009.10.054
Ho YS, Mckay G (1999) Pseudo-second order model for sorption processes. Process Biochem 34:451–465. doi:10.1016/s0032-9592(98)00112-5
Ho YS, Ofomaja AE (2006) Pseudo-second order model for lead ion sorption from aqueous solutions onto palm kernel fiber. J Hazard Mater 129:137–142. doi:10.1016/j.jhazmat.2005.08.020
Hossain MA, Ngo HH, Guo WS, Nguyen TV (2012) Biosorption of Cu(II) from water by banana peel based biosorbent: experiments and models of adsorption and desorption. J Water Sustain 2:87–104
ICdA—Internacional Cadmium Association (2001) Revision of the battery directive: public consultation. ICdA, Bruxelas
International Union Pure and Applied Chemistry (1988) Standard methods for the analysis of oils, fats and derivatives—method 1121. In: Paquot C, Haufenne A (Org.) Determination of moisture and volatiles matter content. Blackwell, Oxford
Iqbal M, Saeed A, Zafar SI (2009) FTIR spectrophotometry, kinetics and adsorption isotherms modeling, ion exchange, and EDX analysis for understanding the mechanism of Cd2+ and Pb2+ removal by mango peel waste. J Hazard Mater 164:161–171. doi:10.1016/j.jhazmat.2008.07.141
Jacques RC, Lima EC, Dias SLP, Mazzocato AC, Pavan FA (2007) Yellow passion-fruit shell as biosorbent to remove Cr(III) and Pb(II) from aqueous solution. Sep Purif Technol 57:193–198. doi:10.1016/j.seppur.2007.01.018
Jimenez RS (2004) Remoção de metais pesados de efluentes aquosos pela zeólita natural escolecita - Influência da temperatura e do pH na adsorção em sistemas monoelementares. Quim Nova 27:734–738. doi:10.1590/s0100-40422004000500011
Khattri SD, Singh MK (2000) Colour removal from synthetic dye wastewater using a bioadsorbent. Water Air Soil Pollut 120(3–4):283–294. doi:10.1023/A:1005207803041
Kumar KV, Porkodi K (2007) Mass transfer, kinetics and equilibrium studies for the biosorption of methylene blue using Paspalum notatum. J Hazard Mater 146:214–226. doi:10.1016/j.jhazmat.2006.12.010
Kurniawan TA, Chan GYS, Lo W, Babel S (2006) Comparison of low-cost adsorbents for treating wastewaters laden with heavy metals. Sci Total Environ 366:409–426. doi:10.1016/j.scitotenv.2005.10.001
Li C, Jiang W, Ma N, Zhu Y, Dong X, Dn Wang (2014) Bioaccumulation of cadmium by growing Zygosaccharomyces rouxii and Saccharomyces cerevisae. Bioresour Technol 155:116–121. doi:10.1016/j.biortech.2013.12.098
Lin SH, Juang RS (2002) Heavy metal removal from water by sorption using surfactant-modified montmorillonite. J Hazard Mater 97(1–3):315–326. doi:10.1016/s0304-3894(02)00026-2
Melo CR (2009). Síntese de zeólita tipo 5a a partir de caulim para adsorção de metais pesados de soluções aquosas. Florianópolis, 89 f. Dissertação (Mestrado em Engenharia Química) – Universidade Federal de Santa Catarina
Meneghel AP, Gonçalves AC Jr, Rubio F, Dragunski DC, Lindino CA, Strey L (2013a) Biosorption of cadmium from water using Moringa (Moringa oleifera Lam.) seeds. Water Air Soil Pollut 224:1383–1396. doi:10.1007/s11270-012-1383-2
Meneghel AP, Gonçalves AC Jr, Strey L, Rubio F, Schwantes D, Casarin J (2013b) Biosorption and removal of chromium from water by using Moringa seed cake (Moringa oleifera Lam.). Quím Nova 36(8):1104–1110. doi:10.1890/s0100-40422013000800005
Mimura AMS, Vieira TVA, Martelli PB, Gorgulho HF (2010) Aplicação da casca de arroz na adsorção dos íons Cu2+, Al3+, Ni2+ e Zn2+. Quim Nova 33:1279–1284. doi:10.1590/s0100-40422010000600012
Montanher SF, Oliveira EA, Rollemberg MC (2005) Removal of metal íons from aqueous solutions by sorption onto rice bran. J Hazard Mater 117:207–211. doi:10.1016/j.jhazmat.2004.09.015
Namasivayam C, Sangeeta D, Gunasekaran DR (2007) Removal of anions, heavy metals, organics and dyes from water by adsorption onto a new activated carbono from Jatropha husk, an agro-industrial solid waste. Process Saf Environ 85:181–184. doi:10.1205/psep05002
Ofomaja AE (2010) Equilibrium studies of copper ion adsorption onto palm kernel fibre. J Environ Manag 91:1491–1499. doi:10.1016/j.jenvman.2010.02.029
Oliveira LS, Franca AS, Ales TM, Rocha SD (2008) Evaluation of untreated cofee husks as potencial biosorbents for treatment of dye-contaminated waters. J Hazard Mater 155:507–512. doi:10.1016/j.jhazmat.2007.11.093
Park D, Yun Y, Park JM (2010) The past, present, and future trends of biosorption. Biotechnol Bioprocess Eng 15(1):86–102. doi:10.1007/s12257-009-0199-4
Pascoal-Neto C, Rocha J, Gil A, Cordeiro N, Esculcas AP, Rocha S (1995) 13C solid-state nuclear magnetic resonance and Fourier transform infrared studies of the thermal decomposition of cork. Solid State Nucl Magn Reson 4:143–151. doi:10.1016/0926-2040(94)00039-F
Pavan FA, Lima EC, Dias SL, Mazzocato AC (2008) Methylene blue biosorption from aqueous solutions by yellow passion fruit waste. J Hazard Mater 150:703–712. doi:10.1016/j.jhazmat.2007.05.023
Pehlivan E, Altun T, Cetin S, Igbal Bhanger M (2009) Lead sorption by waste biomass of hazelnut and almond shell. J Hazard Mater 167:1203–1208. doi:10.1016/j.jhazmat.2009.01.126
Prola LDT (2013) Comparision of Jatropha curcas shells in natural form and treated by non-thermal plasma as biosorbents for removal of reactive red 120 textile dye from aqueous solution. Ind Crop Prod 46:328–340. doi:10.1016/j.indcrop.2013.02.018
Puranik P, Paknikar KM (1999) Influence of co-cations on biosorption of lead and zinc: a comparative evaluation in binary and multimetal system. Bioresour Technol 70:269–276. doi:10.1016/s0960-8524(99)00037-1
Rocha ORS (2012) Evaluation of adsorption process using green coconut mesocarp for removal of reative gray BF-2R dye. Quím Nova 35:1369–1374. doi:10.1590/s0100-40422012000700016
Romero-González J (2005) Determination of thermodynamic parameters of Cr(VI) adsorption from aqueous solution onto Agave lechuguilla biomass. J Chem Thermodyn 37(4):343–347. doi:10.1016/j.jct.2004.09.013
Rubio F, Gonçalves Jr AC, Meneghel AP, Tarley CRT, Schwantes D, Coelho GF (2013a) Removal of cadmium from water using by-product Crambe abyssinica Hochst seeds as biosorbent material. Water Sci Technol 68:227–233. doi:10.2166/wst.2013.233
Rubio F, Gonçalves AC Jr, Dragunski DC, Tarley CRT, Meneghel AP, Schwantes D (2013b) A Crambe abyssinica seed by-product as biosorbent for lead(II) removal from water. Desalin Water Treat 51(1):1–10. doi:10.1080/19443994.2013.836995
Rubio F, Gonçalves AC Jr, Strey L, Meneghel AP, Coelho GF, Nacke H (2013c) Applicability of Crambe abyssinica Hochst. byproduct as biosorbent in the removal of chromium from water. Span J Rural Dev 4(1):25–40. doi:10.5261/2013.GEN1.03
Santhi T, Manonmani S, Smitha T (2010) Removal of malachite green from aqueous solution by activated carbon prepared from the epicarp of Ricinus communis by adsorption. J Hazard Mater 179:178–186. doi:10.1016/j.jhazmat.2010.02.076
Sari A, Tuzen M, Citak D, Soylak M (2007) Equilibrium, kinetic and thermodynamic studies of adsorption of Pb(II) from aqueous solution onto Turkish kaolinite clay. J Hazard Mater 149:283–291. doi:10.1016/j.jhazmat.2007.03.078
Shtangeeva I, Steinnes E, Lierhagen S (2011) Macronutrients and trace elements in rye and wheat: similarities and differences in uptake and relationships between elements. Environ Exp Bot 70:259–265. doi:10.1016/j.envexpbot.2010.09.013
Singha B, Das SK (2011) Biosorption of Cr(VI) ions from aqueous solutions: kinetics, equilibrium, thermodynamics and desorption studies. Colloids Surf B 84:221–232. doi:10.1016/j.colsurfb.2011.01.004
Smith B (1999) Infrared spectral interpretation—a systematic approach. CRC Press, Boca Raton
Sodré FF, Lenzi E, Costa ACS (2001) Applicability of adsorption models to the study of copper behaviour in clayey soils. Quím Nova 24(3):324–330. doi:10.1590/S0100-40422001000300008
Strey L, Gonçalves AC Jr, Schwantes D, Coelho GF, Nacke H, Dragunski DC (2013) Reuse and recycling techniques: Kinetics, equilibrium and thermodynamics of cadmium adsorption by a biosorbent from the bark of Pinus elliottii. In: Bártolo H, Duarte JP (eds) Green design, materials and manufacturing processes, 1st edn. CRC Press Taylor & Francis Group, Boca Raton, pp 433–436
Stuart BH (2004) Infrared spectroscopy—fundamentals and applications. Wiley, Chichester
Tongpoothorn W, Sriuttha M, Homchan P, Chanthai S, Ruangbiriyachai C (2011) Preparation of activated carbono derived from Jatropha curcas fruit shell by simple termo-chemical activation and characterization of their physico-chemical properties. Chem Eng Res Des 89:335–340. doi:10.1016/j.cherd.2010.06.012
Tseng R, Wu CF, Juang SR (2003) Liquid-phase adsorption of dyes and phenols using pinewood-based activated carbons. Carbon 41:487–495. doi:10.1016/S0008-6223(02)00367-6
Vadivelan V, Kumar KV (2005) Equilibrium, kinetics, mechanism, and process design for the sorption of methylene blue onto rice husk. J Colloid Interface Sci 286:90–100. doi:10.1016/j.jcis.2005.01.007
Vaghetti JCP, Lima EC, Royer B, Brasil JL, Cunha BM, Simon NM, Cardoso NF, Noreña CPZ (2008) Application of Brazilian-pine fruit coat as a biosorbent to removal of Cr(VI) from aqueous solution - kinetics and equilibrium study. Biochem Eng J 42:67–76. doi:10.1016/j.bej.2008.05.021
Valdman E, Erijman L, Pessoa FLP, Leite SGF (2001) Continuous biosorption of Cu and Zn by immobilized waste biomass Sargassum sp. Process Biochem 36:869–873. doi:10.1016/S0032-9592(00)00288-0
Wan Ngah WS, Fatinathan S (2010) Adsorption characterization of Pb(II) and Cu(II) ions onto chitosan-tripolyphosphate beads: kinetic, equilibrium and thermodynamic studies. J Environ Manag 91(4):958–969. doi:10.1016/j.jenvman.2009.12.003
Wan Ngah WS, Hanafiah MAKM (2008) Biosorpiton of copper ions from dilute aqueous solutions on base treated rubber (Hevea brasiliensis) leaves powder: kinetics, isotherm, and biosorption mechanisms. J Environ Sci 20(5):1168–1176
Witek-Krowiak A, Szafran RG, Modelski S (2011) Biosorption of heavy metals from aqueous solutions onto peanut shell as a low-cost biosorbent. Desalination 265:126–134. doi:10.1016/j.desal.2010.07.042
Yavuz Ö, Guzel R, Aydin F, Tegin I, Ziyadanogullari R (2007) Removal of cadmium and lead from aqueous solution by calcite. Pol J Environ Stud 16:467–471
Ye M, Caiyan L, Francis G, Makkar HPS (2009) Current situation and prospects of Jatropha curcas as a multipurpose tree in China. Agrofor Syst 76:487–497. doi:10.1007/s10457-009-9226-x
Zhang W, Du Y, Zhai M, Shang Q (2014) Cadmium exposure and its health effects: a 19-year follow-up study of a polluted area in China. Sci Total Environ 470–471:224–228. doi:10.1016/j.scitotenv.2013.09.070
Acknowledgments
This research was supported by the Brazilian National Counsel of Technological and Scientific Development (CNPq) and by the Brazilian Ministry of Science and Technology (MCTI).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Nacke, H., Gonçalves, A.C., Coelho, G.F. et al. Removal of Cd (II) from water using the waste of jatropha fruit (Jatropha curcas L.). Appl Water Sci 7, 3207–3222 (2017). https://doi.org/10.1007/s13201-016-0468-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13201-016-0468-2