Abstract
Water chemistry in the shale bedrock of the Cretaceous-Tertiary of the Cross River and Niger Delta hydrological basins has been investigated using major ions. To carry out a characterization of the water bearing units, 30 and 16 representatives surface and groundwater samples were collected. The evolution of the water is characterized by enhanced content of sodium, calcium and sulphate as a result of leaching of shale rock. The spatial changes in groundwater quality of the area shows an anomalous concentrations of ions in the central parts, while lower values characterize the eastern part of the basin covering Ogoja, Ikom and Odukpani areas. The values of total dissolved solids (TDS) and ions increases down gradient in the direction of groundwater flow. The dissolution of halite and gypsum explains part of the contained Na+, Ca2+, Cl− and SO4 2−, but other processes such as ion exchange, silicate weathering and pyrite oxidation also contribute to water composition. The assessment with contamination indicators such as TDS, hardness, chloride, nitrate and sulphate indicates that the water in area is suitable for human consumption in some locations. Modelling using MINTEQA2 program shows that the water from all the shale water bearing units are under saturated with respect to gypsum.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The Cross River Basin (Nigeria) is often faced with difficulties in supply of water for drinking, domestic and irrigation use. This is partly attributed to the fact that the area is underlain by low permeability shale bedrock. In some cases, most rural people have no fresh drinking water. This scarcity of water seriously threatens the survival of the local population and contributes to poverty and water borne disease. Besides, the water supply in most of these rural communities is almost exclusively through shallow wells and surface water. In addition, the use of water for irrigation makes water a critical resource. Despite its importance, little is known about natural phenomena that govern the chemical composition of water and anthropogenic factors that may affect them.
The natural hydrochemistry of surface and groundwater are principally controlled by the rocks and sediments through which these waters flow through. Background geochemistry is an important tool which can be applied to evaluate the hydrochemistry of water and plan the monitoring of water quality (Cocker 1995; Hook 2005; Pazand et al. 2011). Minerals may influence the chemistry of surface and groundwater through weathering, precipitation, dissolution and ion exchange reactions. This in turn modifies the mineralogy and chemistry of water as it flows on the rock and through the aquifer. More than 60 % of the area of study is underlain by shale which is often problematic hydrogeologically mainly due to low permeability. These problems may also be environmental and geotechnical and in most cases are being influenced by the predominant clay mineral type (Aghamelu et al. 2011). Differences in chemical composition of the different shale bedrock (Nganje et al. under review) appear to influence the chemistry of the waters which drains through these rocks. Most shale aquifer systems in the study area are developed through hand dug well (<20 m deep) and shallow hand pump fitted borehole (<60 m deep).
Within the study area, surface water and groundwater constitute the major source of water supply for domestic, agricultural and industrial purposes. Hence, there is the need to put in place a monitoring programme to continually assess both quantity and quality of these resources for their sustainability. According to Jalali (2007), the greatest threat to maintaining fresh water supply is depletion of the resource that is used to fulfil the needs of the rapidly growing population. Studies by Edet and Ekpo (2008); Nganje et al. (2010) and Edet and Okereke (2014) in parts of the present area of study showed that precipitation through rainfall, water–rock interaction, ion exchange and anthropogenic input are the main controlling factors to water composition.
Considering the importance of water in sustainable development and the severity of the safe drinking water, and the associated environmental problems with shale rocks, it is therefore, pertinent to document the chemistry of the surface and groundwater from the different water bearing units within the shale rocks. This paper, therefore describes the results of a detailed geochemical study of the surface and groundwater in areas underlain by shale bedrock in parts of southeastern Nigeria. The major components of this study were to address the following issues: (1) establish the spatial variability of water composition, (2) determine the major controls on the water composition due to surface and groundwater interactions with underlying shale bedrock, and (3) investigate water quality in the Cross River basin. The data from this work are expected to guide in the design of a long-term monitoring programme to properly manage and protect the different waters bodies within the shale terrain of southern Nigeria by stakeholders.
Location of study area
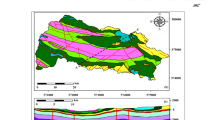
The study area (latitudes 5°04′–6°36′N and longitudes 7°27′–8°48′E) is situated in southeastern Nigeria, between Cameroun border in the east, the lower Benue in the north, and parts of the Benin Basin in the west and Atlantic Ocean in the south (Fig. 1). Elevation in the area varies from less than 85 m around Calabar in the south to about 400 m above sea level at Enugu. The relief of the area is undulating and the major relief structures are hills in Abakaliki area formed by the pyroclastic bodies associated with the shales (Aghamelu et al. 2011).
Location and general geology map of Cross River Basin, Nigeria (Modified from Uma and Onuoha 1991)
The area is characterized by a tropical savanna climate near Enugu in the north to tropical monsoon near Odukpani in the south. The amount of precipitation varies considerably from year to year. The average annual rainfall in Enugu is about 2000 mm and more than 3000 mm south of north of Calabar. Annual average air temperature varies between 25 and 28 °C. The vegetation of the area varies from tropical rainforest in the south to derived savannah in the central and western parts, while savannah dominates in the north. An estimate of the population of Cross River basin based on the 2006 census is 2,000,672 with a population density of 420 people per km2. Within the basin, the main human activities include agriculture, industry, commerce and fishing with agriculture occupying a significant proportion of the landmass. The major crops produced include oil palm, cocoa, rubber, cassava, yam, rice and maize. Agricultural production is still very traditionally oriented.
Geology framework
The regional geology of the area of study has been discussed by several authors (Reyment 1965; Burke 1972; Murat 1972; Olade 1975; Kogbe 1976; Petters and Ekweozor 1982; Fayose 1978). The age of the geological formations extends from Precambrian through Cretaceous to Tertiary with an unconformity from upper Coniacian to lower Campanian. The catchment is composed of sandstone, limestone, shale and marl (Fig. 2). Within the study area, the Albain Asu River Group (Abakaliki Shale considered in this study) is the oldest unit and lie unconformably on the Basement (Oban massif, Obudu plateau). It is composed of bluish grey black and black shales, sandy shales, fine micaceous and calcareous sandstone, and siltstone with limestone intercalations. The Cenomanian Odukpani Formation overlies the Asu River Group and is made of black shales with minor intercalations of limestone and sandstone. The Turonian to early Santonian Eze-Aku Formation consist of black shales intercalated within sandy units and shelly limestones overlie the Odukpani Formation. The Conacian Agwu shale overlies the Eze-Aku shale and is composed of black shale with minor intercalations of limestone and sandstone. Overlying the Agwu Formation is the Campanian to Maastrichtian Enugu/Nkporo Formation consisting mostly of shale, limestone and sandstone. The Imo Formation which is the youngest is found in the Tertiary Niger Delta and constitutes less than 10 % of the area of study. The main rock here is black shale with clay and sandstone intercalations. The Cretaceous sediment was affected by tectonics activities which occurred in two phase folding/faulting episodes in the pre- and post-Turonian times (Nwachukwu 1972). Figure 1 shows the outcrop pattern of geologic formations and major structural elements in the basin. Tectonism was followed by magmatism which resulted in formation of volcanic rocks in the Asu River and Eze Aku Groups. These intrusive rocks have been observed in Obubra and Iyametet (Fig. 3).
Intrusive rocks observed in Obubra area (Location of Obubra is in Fig. 1)
Hydrogeologic framework
Hydrogeologically, the study area falls into three hydrogeological groups of south eastern Nigeria: lower, middle and upper (Uma and Onuoha 1991; Adelana et al. 2008). The lower hydrogeological group is underlain by the predominantly shally Formations of the Abakaliki Shale, Odukpani Shale, Eze-Aku Shale, Agwu Shale, Nkporo Shale and Enugu Shale. The middle hydrogeological group is developed within the Mamu, Ajali and Nsukka Formations, which contain prominent sandy horizons, while the upper hydrogeological group is made up of Imo Shale, Bendi-Ameki, Ogwashi-Asaba and the Benin Formations.
The major feature of the lower hydrogeological group is the occurrence of a thin shallow but extensive unconfined aquifer. The aquifer is formed by the top weathered horizon within the fractured shales and sandy horizons. This aquifer is exploited mainly by hand-dug wells. Groundwater is found in open fractures at shallow depths of between 10 and 40 m. According to MacDonald et al. (2001, 2005a, b), the shales of the Asu River Group are characterized by high transmissivity which is related to the degree of burial and low-grade metamorphism of the shale host rock. The Agwu Shale which is not fractured has low transmissivity thus making it difficult to exploit the aquifer. The saturated thickness is less than 50 m and yields of boreholes are generally less than 0.3 l/s (Uma and Onuoha 1991; Adelana et al. 2008).
The Tertiary Imo Shale of Paleocene to Eocene age belongs to the Upper hydrogeological group comprising of shales, claystones, calcareous mudstones, siltstones, ironstones and lenses of sandstones. The shales are fissile and occasionally interbedded with sandstone intercalations giving rise to localized aquifer–aquitard system. The relation between stratigraphic and hydrogeologic units is presented in Fig. 4.
Groundwater flow system
The groundwater level contour map presented in Fig. 5 summarizes the distribution of hydraulic heads in the area. Groundwater occurs at an average depth of less than 30 m above sea level in the south at Odukpani to more than 100 m at Ohafia in the west under an average hydraulic gradient of 0.02. The general groundwater flow direction is from north to south from Abakaliki anticline into Afikpo syncline. Groundwater also flows from the Ohafia hill into the Okigwe and Odukpani depressions. The flow of groundwater is also from Ikom volcanic hills through Mamfe embayment to Odukpani area from the north to south and northwest to southeast directions.
Materials and methods
Fifty-two samples were obtained from surface water (rain, streams, rivers and ponds) and groundwater (shallow hand dug wells and deep boreholes) sources between July and August 2009 (Fig. 6).
The water samples were collected into clean low-density polyethylene bottles kept in a cooler in the field and were later transferred to a freezer until analysis to avoid microbial activity. The physical parameters including temperature, electrical conductivity (EC), pH, turbidity and dissolved oxygen (DO) were measured in the field using standard field equipment. Sample bottles were rinsed at the sampling site with the water to be sampled before collection. The water samples were filtered using 0.45-μm Millipore filters into sterilized polyethylene bottles. Water collected for major and trace metal analysis were preserved by acidifying with a few drops of HNO3 acid to achieve a pH of ≤2.
Major anions (Cl−, NO3 −, SO4 −, F− and PO4 −) were determined by Dionex 100 Ion Chromatography (IC), equipped with AS4A-AC analytical column, AMMS-11 (4 mm) regenerating suppresser and conductivity detector. The samples were injected through a 12.5-µL sample loop and eluted at 0.5 mL/min using 1.8 ml M Na2CO3 and 1.7 nM NaHCO3. The system was calibrated with prepared standards. For major cations (Ca2+, Mg2+, Na+ and K+) and trace elements (As, Be, Ba, Cr, Cu, Zn and U), the water samples were diluted to ≤1 % before analysis using ICP–MS (Series 200). The anions and cations determinations were carried out at the School of Science, University of West of Scotland, Scotland. Three replicates were run for each sample and the precision obtained in most cases was better 5 % RSD. Statistical analyses were performed by means of the statistical package, STAISTICA (Pilz 1993).
The computer program MINTEQA2 was used to calculate the distribution of the aqueous species in the water. The programme is designed to perform a variety of aqueous geochemical calculations based on ion-association aqueous model. MINTEQA2 has been used to evaluate which solid may be precipitating by means of saturation index. The saturation index is defined as follows: SI = log IAP/K, where IAP is the logarithm of the ions of the solid in solution and K is the solubility product of the solid. If Saturation Index (SI) is zero, the water composition reflects the solubility equilibrium with respect to the mineral phase. A negative value indicates under saturation with respect to the particular mineral and the mineral cannot precipitate from solution, and should dissolve if present, into solution to reach equilibrium concentration (Deutsch 1997). Also, in the case of groundwater with values of SI less than zero indicates water from formation with insufficient quantity of mineral for solution or short residence time (Ako et al. 2011). Values above zero indicate super saturation with respect to the particular mineral phase and incapable of dissolving more of the mineral and water discharging from an aquifer containing sufficient amount of the mineral species with longer residence time to reach equilibrium in the case of groundwater (Ako et al. 2011).
Results
Tables 1 and 2 present detailed results of the physical and chemical parameters in surface and groundwater samples. The statistical summary is presented in Table 3. The data indicate varied composition and reflect variable composition of the recharge and discharge waters in the area.
Physical parameters
Hydrogen ion concentration (pH) and total dissolved solids (TDS) of rain water averaged 7.14 and 21.3 mg/l, respectively, indicating alkaline and fresh nature of rain water.
Temperature of the water samples ranged from 20.2 to 26.0 °C for groundwater and from 20.1 to 26.00 °C for surface water. The temperature is comparable to the ambient local air temperature of the area. pH values varied from 3.31 to 7.73 for groundwater and from 2.95 to 7.59 for surface water. The minimum pH was obtained at location GW 2 within Enugu/Nkporo Shale for groundwater and location SW 25 within the Eze Aku Shale for surface water. The maximum pH values were obtained from Abakaliki Shale at locations GW 11 (groundwater) and SW 29 (surface water). The variation in pH of surface water (mean 5.73) and groundwater pH (mean 6.39) suggests a decrease in CO2 dissolution in groundwater. Fifty percent and 70 % of all the groundwater and surface water samples, respectively, indicate that the water of the study area is acidic (Tables 1, 2). This may be attributed to the oxidation of sulphide minerals and sulphur contained in the shale, while the neutrality of the pH may be due to the buffering effect of limestone associated with the Abakaliki Shale. Turbidity values varied from 0.47 to 127 NTU. The minimum value of turbidity in groundwater was obtained at location GW 12 and the maximum value of 56.78 NTU was obtained at location GW16 (Abakaliki Shale). For the surface water samples, the minimum and maximum values of turbidity were obtained at SW 14 (Enugu/Nkporo Shale) and SW 8 (Imo Shale), respectively. The relative increase in turbidity values in groundwater compared to surface water is attributed to turbid recharging water flowing through the water bearing units. In the case of surface water, high turbidity is due to high sediment load from runoff (Hobbs et al. 1972).
Low variation in dissolved oxygen (DO) recorded for both surface water (5.32–6.62 mg/l) and groundwater (4.18–6.42 mg/l) are expected. According to Boughton and McCoy (2006), oxygen is supplied to groundwater through recharge water and by movement of air through unsaturated zone. Electrical conductivity (EC) of the water samples varied from 28 µs/cm at location GW 11 (Abakaliki Shale) to 1315 µs/cm at location GW 1 (Imo and Abakaliki Shale) for groundwater. For surface water, EC varied from 5.60 at location SW 29 (Abakiliki Shale) to 2720 µs/cm at location SW 25 (Odukpani Shale). The variation in EC is attributed to the different levels of enrichment in depositional environment during accumulation (Rimmer 2004).
Characteristics of cations
The dominant cation is Ca2+ and the order of abundance is Ca2+ > Mg2+ > Na+ > K+ for both surface and groundwater. The level of Na+ in groundwater varied from 2.38 (location GW 9, Eze Aku Shale) to 44.53 mg/l (location GW 13 (Abakaliki Shale), while the concentration in surface water ranged between 1.6 (SW 8, Imo Formation) and 11.65 mg/l (location SW 30, Abakaliki Shale). The level of K+ in groundwater varied between 0.38 at location GW 3 (Eze Aku Formation) and 26.83 mg/l at location GW 10 (Abakiliki Shale). The concentration of K+ in the surface water varied between 0.97 at SW 4 (Abakaliki Formation) and 13.56 mg/l at SW 17 (Eze Aku Shale). The level of Ca2+ ranged from 6.5 mg/l location GW 9 (Imo aquitard) to 83.3 mg/l at GW 15 (Abakaliki Formation), whereas for surface water, Ca2+ ranged between 0.79 at SW 23 (Odukpani Formation) and 274.10 mg/l at location SW 25 (Odukpani Shale). Magnesium ion (Mg2+) in groundwater varied from 1.19 mg/l (location GW 3, Enugu/Nkporo Shale) to 14.14 mg/l (location GW 2 (Enugu/Nkporo Shale). In surface water, Mg2+ concentration ranged between 0.3 (SW 2, Eze Aku Shale) to 34.56 mg/l (location SW 25, Odukpani Shale).
With the exception of K+ (Eze Aku Shale, surface water) and Mg2+ (groundwater, Enugu/Nkporo Shale), high proportion of alkaline earth metals (Ca2+and Mg2+) relative to alkali metals (Na+ and K+) reflect the effect of dissolution of minerals such as pyroxene, calcite, gypsum, anhydrite and dolomite (Wanty et al. 2009). The sources of these minerals are associated with limestone, marl, dolerite and pyroclastic materials associated with the shale rocks in the study area. The high concentration of Ca+ for all the water bearing units may probably be due to water–rock interaction as most of the rocks contain mineral species such as calcite, gypsum and anhydrite. The low level of K+ relative to Ca2+, Mg2+ and Na+ may be due to the fact that it can easily be fixed by clay minerals (Hem 1992).
Characteristics of anions
Nitrate and sulphate constituted the dominant anions measured in both the surface water and the groundwater. The concentrations of Cl− and HCO3 − were generally below the detection limit of the equipment (BDL) or negligible in the water. The level of SO4 2− varied between BDL at locations GW 9, GW 11 and GW 13 (Enugu/Nkporo Shale) to 8542.80 mg/l at location GW 2 (Enugu/Nkporo Shale) for groundwater. For surface water, the minimum value of BDL was obtained at locations SW 12, SW 28, SW 29 and SW 30 and maximum value of 27594.30 mg/l at location SW 25. The maximum value of sulphate in surface water was from the Odukpani Formation. The source of SO4 may be due to oxidation and dissolution of sulphur from pyrite contained in the shale. Other probable sources of elevated sulphate include gypsum dissolution, use of manure, artificial fertilizers and leaching of acid sulphate soils (Edet et al. 2012).
With the exceptions of groundwater samples from Imo, Enugu/Nkporo Shale and Abakaliki Shale, the values of nitrate (Table 2) obtained for all the water bearing units were below 50 mg/l, the standard set by WHO (1993) for drinking and domestic purposes. The levels of nitrate in groundwater varied between BDL at location GW 13–86.96 mg/L at GW 2 in Enugu/Nkporo Formation (Table 2). In respect of surface water, nitrate values varied from BDL at SW 2, SW 13, SW 15, SW 16, SW 19, SW 25 and SW 29–230 mg/at SW 10 (Enugu/Nkporo Shale). The relatively high values for nitrate obtained for some groundwater samples (GW 1, GW 2, GW 3, GW 6, GW 10 and GW 12) are attributed to application of nitrogen fertilizer in the cultivation of crop plants and irrigation of the farmland by the use of sewage effluent and poor quality surface water runoff and infiltration into the groundwater system, especially the shallow hand dug wells. Generally, the spatial variation of anions was attributed to the level of influence of human activities as well as relative enrichment among different shale formations.
Spatial variations of ions in groundwater
The spatial changes in groundwater quality of the area are illustrated in Figs. 7, 8, 9, 10 and 11. The total dissolved solids (TDS) distribution map (Fig. 7) shows variation between 100 and 400 mg/l. An anomalous area with values greater than 400 mg/l occurs in the central part near Afikpo and in the southwest at Umuahia. The spatial distribution of combine sodium and potassium (Fig. 8) shows one anomaly towards the central part characterized by a contour of 60 mg/l. The sodium + potassium ions show conformity with the TDS distribution map. Lower Na+ + K+ values (<20 mg/l) characterize eastern parts of the basin covering Ogoja, Ikom, Obubra and Odukpani. The combine calcium and magnesium also show anomalous concentration (>60 mg/l) in the central part (Fig. 9) and in conformity with Na + K and TDS distribution maps. The anomaly is elongated in the northeast/southwest direction. Lower values (<20 mg/l) are recorded in the northeastern parts of the area. The spatial distribution of sulphate (Fig. 10) show lower (<1000 mg/) in the northeast with higher values (>4000 mg/l) in the south. The values increase down gradient in the direction of groundwater flow. Nitrate is linked to the quality of groundwater in the area. The values of nitrate show anomalous concentration (>40 mg/l) in the central parts increasing gradually to higher values (>60 mg/l) in the down gradient (Fig. 11) and in groundwater flow direction.
Spatial distribution of total dissolved solids (TDS) in groundwater of the study area (chemical data in Table 1)
Spatial distribution of Na+ + K+ in groundwater of the study area, (chemical data in Table 1)
Spatial distribution of Ca2+ + Mg2+ in groundwater of the study area (chemical data in Table 1)
Spatial distribution of SO4 2− in groundwater of the study area (chemical data in Table 1)
Spatial distribution of NO3 − in groundwater of the study area (chemical data in Table 1)
Drinking water quality
The assessment of water quality was made using contamination indicators and comparing the concentrations of these indicators with standards. These indicators include total dissolved solids (TDS), total hardness (TH), chloride, nitrate and sulphate.
To ascertain the suitability of the water samples for any purpose, the samples were classified according to their TDS values (Freeze and Cherry 1979), which are presented in Table 4. The surface water is fresh water except a few samples representing brackish water. All the groundwater samples had TDS < 1000 mg/l, indicating low content of ions which can be used for drinking without any risk.
The classification of the water samples (Table 4) based on total hardness (TH) shows that majority of the samples are soft based on the classification given by Sawyer and McMcartly (1967). However, 18.8 % each of the groundwater samples were moderately hard and hard, while 20 % of the surface water samples were moderately hard. For the same surface water samples, about 16.7 % was very hard.
In terms of chloride content, all the water samples were below the most desirable limits (<200 mg/l), indicating good quality water for drinking purpose.
The concentration of nitrogen in water is derived from the biosphere (Saleh et al. 1999). Nitrogen is originally fixed from the atmosphere and then mineralized by bacteria to ammonium. In aerobic conditions, nitrogen is converted to nitrate by nitrifying bacteria (Tindall et al. 1995). 75 and 83.3 % of the groundwater (GW) and surface water (SW) had nitrate concentrations within the most desirable limit (<45 mg/l). However, 25 and 16.7 % exceeded this value (>45 mg/l) for GW and SW, respectively. The high concentration of nitrate in some water samples is toxic and may cause blue baby disease in children (Comly 1945; Gily et al. 1984). The high concentration of nitrate in some locations may be due to application of fertilizer and poor waste management.
Sulphate is unstable if it exceeds the maximum allowable limit of 400 mg/l and causes laxative effect on human system with excess magnesium in water (Subbramani et al. 2005). From Table 4, 62.5 % of GW and 70.0 % of SW exceed the maximum allowable limit of sulphate for drinking water. Considering the positive correlation of sulphate and magnesium (Table 5), this may result in gastrointestinal irritation to the human system.
Agricultural water quality based on chloride and sulphate content
Chloride and sulphate have been used for assessment of suitability of water for agriculture (Sagnak 1991; Bauder et al. 2004; Hopkins et al. 2007). Chloride is an essential element for plant and also important criterion for irrigation water. Sulphate is necessary for plant nutrition; however, water containing more than 1000 ppm of sulphate has disadvantage for plants (Sagnak 1991). It was observed that all the 4 rain water samples with respect to chloride concentration fall in the excellent class (<142 mg/l), Table 5. For sulphate concentration, 4 samples fall into excellent class, while 8 samples fall in the permissible and precaution useable class and only 4 samples in the unsuitable class for groundwater. For the surface water samples, all the 4 rain water samples fall in the excellent class in addition to 8 samples from other surface water sources. Three surface water samples are considered to be in good and permissible class, while 7 samples fall in the precaution useable class. Only 12 samples are considered to be unsuitable (Table 5).
Discussion
Chemical facies
The proportion between main cations and anions are presented in the form of a Piper diagram (Piper 1944) in Fig. 12. Major cations reveal similar proportions in the area, intermediate between Ca2+ and Na+ content. Results of chemical analyses indicate enrichment in SO4 2− relative to Cl− and HCO3 − except sample GW 9 which is depleted in SO4 2− relative to Cl− ions. Majority of the water type fell in the field calcium-sulphate (Ca2+–SO4 2−). This water type made up 92 and 83 % of groundwater and surface water. This was followed by sodium-sulphate (Na+–SO4 2−) types, which made up 8 % of groundwater and 17 % of surface water. Abundance of Ca2+–SO4 2− water type is probably the result of dissolution of gypsum mineral from the rock matrix. The source(s) of other water types is due to minor variations in the lithology of the bedrock.
Acidity in water
The Pearson’s correlation coefficient (r) was used to determine the relationship between the dissolved ions and their possible sources. The Pearson’s correlation coefficient (r) is based on the method of covariance. It is a unit less number, which ranges between +1 and −1, where +1 indicated a perfect direct relationship between two variables and a correlation of −1 indicated an inverse perfect relationship. Between the two extremes is a spectrum of less-than-perfect relationships, including zero, which indicated lack of linear relationship. High degree of correlation exists between two variables if the coefficient value lies between ±0.50 and ±1, then it is said to be a strong correlation. If the values of r lies between ±0.30 and ±0.49, then the correlation is moderate (Davis 1986).
Pearson’s correlation value (Table 5) shows a negative relation between conductivity (EC) and pH since elements are more ionized at low pH values. Thus, concentration of ions increases with increasing conductivity of water as pH is reduced or as the acidity increases. The relationship between EC and ions has been used to identify evaporation processes (Kumar et al. 2006). Besides, relation between EC and SO4 2− (Table 6) indicates that SO4 contributes to the modification of water chemistry. The relation between pH and Mg (Table 6) suggests that the dissolution of Mg (silicates) contributes to the pH or the increase in pH is probably associated with the precipitation of Mg. Correlation between pH and SO4 shows a reverse relationship as expected indicating that SO4 is contributing to the acidity of the water.
Atmospheric input and water–rock interaction
Three main sources of dissolved ions into inland waters include the following: (1) atmospheric deposition of salts, (2) weathering of rocks forming minerals and (3) anthropogenic input (Singh et al. 2005; Berner and Berner 1987; Zhang et al. 1995; Sarin and Krishnaswamy 1984; Singh and Hasnain 1998, 1999, 2002). An estimate of the atmospheric contribution to the aquatic system can be assessed by comparing the chemical composition of the surface and groundwater with that of the rain water in the study area as stipulated by Pandey et al. (1994) and Sarin et al. (1989). The local rain water collected from study area has been used to derive the atmospheric contributions (Table 7). The assessment show high concentration (>50 %) atmospheric contribution of Na+, K+ (groundwater) Na+, K+, Ca2+ and Mg2+ (surface water) and low concentration (<50 %) for Ca2+, Mg2+ SO4 2+ and NO3 − (groundwater) and SO4 2+ and NO3 − (surface water). This indicates weathering of the basin and anthropogenic sources for these ions. The relative high contribution of Na+, K+, Ca2+ and Mg2+ is due to opencast salting mining, dust from cement manufacturing plants and limestone quarries. NO3 − may be due to atmospheric and pollution sources (Jeong 2001). A plot on a modified Gibbs diagram (Gibbs 1970) places most of the water samples in the region of rock dominance, indicating rock weathering as a primary factor controlling the water composition (Fig. 13).
The Geochemical processes occurring within surface and groundwater and reactions with aquifer material have a great effect on water quality (Herczeg et al. 1991), thus the evaluation of the importance of such processes is essential if the water resources are to be properly developed for human consumption, agricultural and industrial activities. The concentration of dissolved solute in water is controlled by several process such as weathering, dissolution and ion exchange (Panopaulos et al. 2004; Kumar et al. 2006; Garcia et al. 2001; Singh et al. 2005; Edet and Ekpo 2008; Nganje et al. 2010). In the present work, various cross plot relations were used to evaluate the processes responsible for the variation in the chemistry of the water in the study area. Therefore, in order to specify the likely origin of each major element contributing to groundwater mineralization, plots of Na+ versus Cl− and Ca2+ versus SO4 2− content are shown in Figs. 14 and 15. Na+ vs Cl− relationship has often been used to identify mechanisms responsible for the origin of water salinity (Magaritz et al. 1981; Dixon and Chiswell 1992; Guendouz et al. 2002). The relationship between these ions shows that four out of five data points line above the line of slope 1 (halite dissolution), while one lies below the halite dissolution line. This reflects two scenarios. First scenario is most likely the release of Na+ by feldspar weathering via reactions such as:
Equation above also produces kaolinite, which is common in the study area. The second scenario of lower Na+/Cl− ratio probably reflects the cation exchange reactions leading to adsorption of Ca2+ on clay minerals and simultaneous releasing of Na+ ions. Those samples in which the Na+/Cl− molar ratios are higher than one (Table 8) also show a deficiency in Ca2+ with respect to SO4 2− ions.
Bivariate plot of Na+ versus Cl− values in groundwater of the area (chemical data in Table 1)
Bivariate plot of Ca2+ versus SO4 2− values in groundwater, rainwater and surface water of the area (chemical data in Table 1)
On the other hand, the Ca2+ versus SO4 2− plot (Fig. 15) showed more pronounced loss of Ca2+ with respect to SO4 2−. This may be due to calcite precipitation controlled by gypsum dissolution which tends to maintain saturation or oversaturation with calcium bearing minerals (Abid et al. 2011). For the study area, fractions of cations derived from evaporites complexes with Cl− are likely to be insignificant since (1) the area has low Cl and high Na+/Cl− ratio (Table 7) and (2) the water samples are characterized by low Mg2+/Na+ ratios (Tables 8, 9), whereas input from evaporites will have very low (<0.2) Mg/Na ratios (Negrel et al. 1993). Weathering of silicates and carbonates may also contribute to the chemistry of water in the area. Water draining only carbonates show Ca2+ and Mg2+ dominated reservoirs and Ca2+/Na+ ratios close to 50 and Mg2+/Na+ close to 10 (Negrel et al. 1993; Meybeck 1986; Stallard 1980). The chemical composition assigned for silicate end member is Ca2+/Na+ = 0.35 ± 0.15 and Mg2+/Na+ 0.24 ± 0.12 (Gaillardet et al. 1999). The observed ratios for Ca2+/Na+ and Mg2+/Na+ are much lower than those for carbonate rocks and close to those draining silicate rocks indicating that the dissolved chemistry of water in the area is essentially controlled by silicate weathering in addition to gypsum dissolution. The concentration of sulphate content and high SO4 2−/Ca2+ ratios are probably controlled by water–rock gypsum dissolution and pyrite reduction via a reaction below:
It is revealed by surface mapping that most of the shale rocks contain pyrite minerals. This is supported by negligible concentrations of bicarbonate because of low pH and high SO4 2−/Cl− ratios (Hounslow 1995).
The relationship between Ca versus Mg is usually investigated to determine the contribution of calcite and dolomite to water composition (Kumar et al. 2006). In this study, the plot of Ca vs Mg (Fig. 16) indicates that most of the samples lie above the equiline line and only a few surface water samples are on the equiline indicating contribution from dolomite dissolution. The excess of Ca indicates calcite dissolution or calcite-rich minerals that contribute Ca to the water, i.e. dissolution of silicate minerals (Ettazarini 2005; Kumar et al. 2006) as shown below (Pawar et al. 2008):
The relationship between Na+ and K+ (Fig. 17) could conform to ion exchange process. The excess of Na+ over K indicates that Mg2+ and Ca2+ are captured by Na+-rich clays of the shale. The Na+ is then returned to the aqueous system as against K+ which is known to be relatively immobile (Hem 1992). Other sources of Na and K may be weathering of silicate minerals such as Albite and K-feldspar as shown in Eq. 5 (Pawar et al. 2008):
Bivariate plot of Ca2+ versus Mg2+ values in groundwater, rain water and surface water of the area (chemical data in Table 1)
Bivariate plot of Na+ versus K+ values in groundwater, rainwater and surface water of the area (chemical data in Table 1)
Modelling
Mineral equilibrium calculations for water are useful in predicting the presence of reactive minerals in groundwater system and estimating mineral reactivity (Deutsch 1997). By using the saturation index (SI), it is possible to predict the reactive mineralogy (Deutsch 1997). In the present study, SI of gypsum was calculated to determine equilibrium between mineral and water. The calculated SI for gypsum ranged from −1.80 to −1.0 with average of −1.50 for groundwater and −3.2 to −0. 91 with an average of −1.75 for surface water samples. All the groundwater and surface water were below the equilibrium state for gypsum. Generally, SO4 2− and OH− constitute the major dissolved species in this water.
Conclusions
The shale rocks of the Cross River and Niger Delta hydrological basins are one of the most important water resources in southeastern Nigeria. It is the main water source used for drinking, domestic and agricultural purposes by the local population. Therefore, determining the source and mechanism controlling the water chemistry outlined in this study is of great importance. Hence, analysis of the major ions in surface and groundwater was carried out.
The groundwater map points to the significant role of the Abakaliki Anticline, Ikom and Ugep Hills in local groundwater flow direction and recharge of the aquifer. The principal changes in the chemical composition of the water results from halite dissolution, silicate weathering, as well as ion exchange and pyrite oxidation. Generally, mineralization of groundwater increases along the groundwater flow direction from Abakaliki Anticline to Afikpo Syncline and from Ikom and Ugep Hill to Odukpani area.
The dissolution of halite and gypsum explains part of the contained Na+, Ca2+, Cl− and SO 24 -, but other processes such as ion exchange, silicate weathering and pyrite oxidation also contribute to water composition. The Na+/Cl− and Ca2+/SO4 2− ratios suggest dissolution of shale rocks. The value of Na+/Cl− ratio higher than 1 indicates occurrence of ion exchange releasing Na+ into water and simultaneous removal of Ca2+. The existence of such exchange is confirmed by Na+/K+ and Na+/Cl− ratios. Chemical data indicate the importance of pyrite oxidation as a factor controlling water chemistry. Occurrence of low saline waters suggests local recharge of the aquifers by recent rain and surface water. The spatial changes in groundwater quality of the area show an anomalous concentration of ions in the central parts, while lower values characterize the eastern parts of the basin covering Ogoja, Ikom, Obubra and Odukpani. The concentration of TDS and ions increases down gradient in the direction of groundwater flow. The assessment of contamination indicators such as TDS, hardness, chloride, nitrate and sulphate indicates that the water in area is suitable for human consumption in some locations. Modelling using MINTEQA2 program shows that the water from all the shale rocks is under saturated with gypsum.
References
Abid K, Zouari K, Dulinski M, Chkir N, Abidi B (2011) Hydrologic and geologic factors controlling groundwater chemistry in the Turonian aquifer (southern Tunisia). Hydrogeol J 19:415–427
Adelana SMA, Olasehinde PI, Bale RB, Vrbka P, Edet AE, Goni IB (2008) An overview of the geology and hydrogeology of Nigeria. In: Adelena S, MacDonald A (eds) Applied groundwater studies in Africa: IAH selected papers on hydrogeology, vol 3. pp 171–198
Aghamelu OP, Nnabo PN, Ezeh HN (2011) Geotechnical and environmental problems related to shales in the Abakaliki area, Southeastern Nigeria. Afr J Environ Sci Tech 5(2):80–88
Ako AA, Shimada J, Hosono T, Ichiyanagi K, Nkeng GE, Fantong WY, Eyong GET, Roger NN (2011) Evaluation of groundwater quality and its suitability for drinking and agricultural uses in the Banana Plain ( Mbanqa, Njombe, Penja) of Cameroon Volcanic Line. Environ Geochem Health 33:559–575
Bauder TA, Waskom RM, Davis JG (2004) Irrigation water quality criteria. Colorado State University, Cooperative Extension, Factsheet No. 0.506. http://uwadmnweb.uwyo.edu/soilfert/Pubs/Irrigation%20water%20quality20CSU.pdf
Berner EK, Berner RA (1987) The global water cycle: geochemistry and environment. Prentice-Hall, Englewood Cliffs
Boughton CJ, McCoy KJ (2006) Hydrogeology, aquifer geochemistry and groundwater quality in Morgan county, west virgina. US Geological Survey Scientific Investigation Report 2006–5198:56
Burke K (1972) Longshore drift submarine fans in the development of the Niger Delta. Am Assoc Pet Geol Bull 55:1975–1983
Cocker MD (1995) Geochemistry and hydrochemistry of the Oconee River Basin. In: Hatcher KJ (ed) Proceedings of 1995 Georgia water resources conference held on the 11–12 April 1995, University of Georgia pp 67–70
Comly HH (1945) Cyanosis in infants caused by nitrates in well water. J Am Mwd Assoc 129:12–114
Davis JC (1986) Statistics and data analysis in geology. Wiley, New York
Deutsch WJ (1997) Groundwater geochemistry fundamentals and applications to contamination. Lewis, New York
Dixon W, Chiswell B (1992) The use of hydrochemical sections to identify recharge areas and saline intrusions in alluvial aquifers, southeast Queenland Australia. J Hydrol 130:299–388
Edet AE, Ekpo BO (2008) Hydrogeochemistry of a fractured aquifer in the Ogoja/Obudu area of SE Nigeria. Hydrogeology 13:391–403
Edet A, Okereke CS (2014) Hydrogeologic framework of the shallow aquifers in the Ikom Mamfe Embayment, Nigeria using an integrated approach. J Afr Earth Sci 92:25–44
Edet AE, Worden RH, Mohammed EA, Preston MR (2012) Hydrogeochemical processes in shallow coastal sand aquifer and tidal river systems (Calabar, Southeastern Nigeria): tracking wastewater and seawater pollution in ground and river waters. Environ Earth Sci. doi:10.1007/s12665-011-1275-9
Ekwueme BN, Nyong EE, Petters SW (1995) Geological excursion guidebook to Oban massif, Calabar Flank and Mamfe Embayment, southeastern. Decford publishimg compamy, Calabar
Ettazarini S (2005) Processes of water-rock interaction in the Turonian aquifer of Oum Er-Rabia Basin. Morocco Environ Geol 49:293–299
Fayose EA (1978) Depositional environments of Carbonates, Calabar Flank, southeastern Nigeria. J Mining Geol 15:1–13
Freeze RA, Cherry JA (1979) Groundwater. Prentice Hall, Englewood Cliffs
Gaillardet J, Dupre B, Louvat P, Allegre CJ (1999) Global silicate weathering and CO2 consumption rates deduced from the chemistry of large rivers. Chem Geol 59:3–30
Garcia MG, Hidalgo MDV, Blesa MA (2001) Geochemistry of groundwater in the alluvial plain of Trucman province, Argentina. Hygrolgeo J 9:597–610
Gibbs RJ (1970) Mechanism controlling world water chemistry. Science 17:1088–1090
Gily G, Corrao G, Favilli S (1984) Concentrations of nitrates in drinking water and incidence of gastric carcinomas. First descriptive study of the Piemonate Region Italy. Sci Total Environ 34:35–37
Guendouz A, Moulla AS, Edmunds WM, Zouari K, Shand P, Mamou A (2002) Hydrogeochemical and isotropic evolution of water in the complexe Terminal aquifer in the Algerian Sahara. Engineering Sciences Faculty, Blida, Algeria pp 483–495
Hem JD (1992) Study and interpretation of the chemical characteristics of natural water. USGS water supply paper, p 2254
Herczeg AL, Torgersen T, Chivas AR, Habermehl MA (1991) Geochemistry of groundwaters from the great Artesian Basin Australia. J Hydrol 126(1991):225–245
Hobbs WA, Fried EA, Chistolin JL (1972) Water resources of the Potomac River Basin, West Virginia: West Virginia Geological and Econmic Survey. River Basin Bull 3:110
Hook Z (2005) An assessment of the quality of drinking water in rural districts in Zimbabwe. The case of Gokwe South, Nkayi Lupene and Nwenezi districts. Phys Chem Earth 30:859–866
Hopkins BG, Horneck DA, Stevens RG, Ellsworth JW (2007) Irrigation water quality in the Pacific Northwest, PNW 597-E Pacific Northwest Extension, Oregon State University, University of Idaho, Washington State University. http://extension.oregonstate.edu/catalog/pdf/pnw/pnw597-e.pdf
Hounslow AW (1995) Water quality data: analysis and interpretation Lewis Publishers, New York, p 397
Jalali M (2007) Hydrochemical identification of groundwater resources and their changes under the impacts of human activity in the Chah Basin in western Iran. Environ Monit Assess 130:347–364
Jeong CH (2001) Effect of landuse and urbanization on hydrochemistry and contamination of groundwater from Taejon area, Korea. J Hydrol 253:194–210
Kogbe CA (1976) The Cretaceous and Paleocene sediments of Southern Nigeria. In: Kogbe CA (ed) Geology of Nigeria. Elizabethan Press, Lagos, pp 273–282
Kumar M, Ramanathan A, Rao SO, Kumar B (2006) Identification and evaluation of hydrogeochemical processes in the groundwater environment of Delhi, India. Environ Geol 50:1025–1039
MacDonald AM, Davis I, Peart RJ (2001) Geophysical methods for locating groundwater in low permeability in sedimentary rocks. Examples from Southeast Nigeria. J Afr Earth Sci 32:115–131
MacDonald AM, Kemp SJ, Davis J (2005) Transmissitivity variants in mudstone. Groundwater 49:259–269
MacDonald AM, Lobbing J, Davis J (2005) Developing Groundwater for rural water supply in Nigeria. British Geology Survey Commissioned Report CROS/219N
Magaritz M, Nadler A, Koyumdjisky H, Dan N (1981) The use of Na/Cl ratio to trace solute sources in a semiarid zone. Water Resour Res 17:602–608
Meybeck M (1986) Compositiondes ruiseau non pollues de France. Sci Geo Bull 39:3–77
Murat RC (1972) Stratigraphy and Palaeogeography of the Cretaceous and lower Tertiary in Southeastern Nigeria In: Desauvagie TFJ, Whiteman AJ (eds) African Geology Ibadan, Nigeria, University of Ibadan press, p 251–266
Negrel P, Allegre CJ, Dupre B, Lewin E (1993) Erosion sources determined by inversion of major and trace element ratios and strontium isotropic ratios in river water: the Congo Basin case. Earth Planet Sci Lett 120:59–76
Nganje TN, Adamu CI, Ugbaja AN, Amah AE (2010) Evaluation of hydrogeochemical characteristics of groundwater in parts of Lower Benue Trough Nigeria. Chin J Geochem 29:398–406
Nganje TN, Hursthouse AS, Edet A, Stirling D, Adamu CI (2014) Assessment of the health risk, aesthetic and agricultural quality of rainwater, surface water and groundwater in the shale bedrock areas. Water Quality Expo Health, Southeastern Nigeria. doi:10.1007/s12403-014-0136-4
Nwachukwu SO (1972) The tectonic evolution of southern portion of the Benue Trough Nigeria. Geol Mag 109:411–419
Olade MA (1975) Evaluation of Nigerian’s Bemue Trough (aulacogen): A tectonic model. Geol Mag 12:575–583
Pandey K, Sarin MM, Trivedi JR, Krishnaswami S, Sharma KK (1994) The Indus River system (India-Pakistan): major-ion chemistry, uranium and strontium isotopes. Chem Geol 116:245–259
Panopaulos G, Lambrakis N, Tsolis-Katagas P, Papoulis D (2004) Cation exchange processes and human activities in unconfined aquifer. Environ Geol 46:542–552
Pawar NJ, Pawar JB, Kumar Supekar S (2008) Geochemical eccentricity of groundwater allied to weathering of Basalts from the Deccan volcanic Province, India: Insinuation on Co2 consumption. Aquat Geochem 14:41–71
Pazand K, Gbanbari Y, Aghavali, Hezarkhani A (2011) Groundwater geochemistry in the middle Meshkinshahr basin of Ardabil province in Iran. Environ Earth Sci. doi:10.1007/s12665-011-1131-8
Petters SW (1982) Central West African Cretaceous—Tertiary benthic Foraminifera and stratigraphy. Paloeontographica A 179:1–104
Petters SW, Ekweozor CM (1982) Petroleum geology of Benue Trough and Southeastern Chad Basin Nigeria. American Association of Petroleum Geologist (AAPG), USA
Pilz J (1993) Statistica for windows. Statsoft Inc
Piper AM (1944) A graphical procedure in the geochemical interpretation of water analysis. Am Geophys Union Trans 25:914–928
Ramanathan RM, Fayose EA (1990) Cretaceous trangressions and regressions in Calabar Flank, SE Nigeria. In: Ofoegbu CO (ed) The Benue Trough, pp 60–75
Reyment RA (1965) Aspects of Geology of Nigeria. Ibadan University Press, Ibadan
Reyment RA (1980) Paleo-oceanography and paleo-biogeography of Creataceous South Atlantic Ocean. Oceanologica Acta 3:127–133
Rimmer SM (2004) Geochemical paleoredox indicators in Devonian-Mississippian black shales, Central Appalachian Basin (USA). Chem Geol 206:373–398
Sagnak C (1991) Groundwater pollution originated from geological formation: example of Konya-Çumra-Karapinar plain with GIS application. Department of Geotechnical Services DSI, Ankara
Saleh A, Al-Ruwaih F, Shehata M (1999) Hydrogeochemical processes operating within the main aquifers of Kuwait. J Arid Environ 42:195–209
Sarin MM, Krishnaswamy S (1984) Major ion chemistry of the Ganges and Brahmaputra river systems, India. Nature 312:538–541
Sarin MM, Krishnaswamy S, Dilli K, Somajajulu BLK, Moore WS (1989) Major ion chemistry of the Ganga-Brahmaputra and fluxes to the Bay of Bengal. Geochem Cosmochim Acta 53:997–1009
Sawyer GN, McMcartly DL (1967) Chemistry of sanitary engineers, 2nd edn. McGraw Hill, New York 518
Singh AK, Hasnain SI (1998) Major ion chemistry and control of weathering in high altitude basin, Alaknanda, Garhwal, Himalaya, India. Hydrol Sci J 43:825–845
Singh AK, Hasnain SI (1999) Environmental geochemistry of Damodar river basin-east coast of India. Environ Geol 37:124–136
Singh AK, Hasnain SI (2002) Aspects of weathering and solute acquisition processes controlling chemistry of sub-alpine proglacial streams of Garhwal Himalaya, India. Hydrol Process 16:835–849
Singh AK, Mondal GC, Singh PK, Singh S, Singh TB, Tewary BK (2005) Hydrochemistry of reservoirs of Damodar River basin, India: weathering processes and water quality assessment. Environ Geol 48:1014–1028
Stallard RF (1980) Major element geochemistry of Amazon River system. PhD Thesis, WHOI-80-29, MIT/Woods Hole Oceanogr Inst
Subbramani T, Elagngo L, Damodarasamy SR (2005) Groundwater quality and its suitability for drinking and agricultural use in Chithar River Basin, Tamil Nadu, India. Environ Geol 47:1099–1110
Tindall JA, Petrusak RL, McMohan PB (1995) Nitrate transport and transformation process in unsaturated porous media. J Hydrol 169:51–94
Uma KO, Onuoha KM (1991) Groundwater resources of the Lower Benue Trough, Southern Nigeria. In: Offeagbu CO (ed) the Benue Trough, pp 77–91
Wanty RB, Verplank PL, San Juan CA, Church SE, Schmidt TS, Fey DL, Dewitt EdH, Klein TL (2009) Geochemistry of surface water in alpine catchments in central Colorado, USA: resolving host-rock effects at different spatial scales. Appl Geochem 24(2009):600–610
WHO (1971) International standards for drinking water. World Health Organisation, Geneva
WHO (1983) Guidelines for drinking water quality. World Health Organisation, Geneva, p 186
WHO (1993) Guidelines for drinking water quality. vol 1, Recommendations, World Health Organisation, Geneva, Switzerland
Zhang J, Huang WW, Letolle R, Jusserand C (1995) Major element chemistry of the Huangbe (Yellow river), China-weathering processes and chemical fluxes. J Hydrol 168:173–203
Acknowledgments
Funding for this research work was provided by the Commonwealth Scholarship Commission in the United Kingdom (NGCF 2009-154). The contributions of Prof CS Okereke of the Department of Geology, University of Calabar is also much appreciated. Messrs Anthony Ekpo and Udoh Innocent Mathew assisted in the fieldwork. Dr H. Ezeh, Mr P. Nnabo and Besong Elizabeth provided logistics support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Nganje, T.N., Hursthouse, A.S., Edet, A. et al. Hydrochemistry of surface water and groundwater in the shale bedrock, Cross River Basin and Niger Delta Region, Nigeria. Appl Water Sci 7, 961–985 (2017). https://doi.org/10.1007/s13201-015-0308-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13201-015-0308-9