Abstract
Leachate generated by open solid waste disposal sites contains substances likely to contaminate groundwater. The impact of potential contaminants migrating from leachate on groundwater can be quantified by monitoring their concentration and soil properties at specific points in the unsaturated zone. In this study, physical and chemical analyses were carried out on leachate, soil and water samples within the vicinity of the municipal solid waste disposal site at Abloradjei, a suburb of Accra, Ghana. The area has seen a massive increase in population and the residents depend on groundwater as the main source of water supply. Results obtained indicate alkaline pH for leachate and acidic conditions for unsaturated zone water. High EC values were recorded for leachate and unsaturated zone water. Major ions (Ca2+, Na+, Mg2+, K+, NO3 −, SO4 2−, Cl−, PO4 3− were analysed in leachate, unsaturated zone water, soil solution and groundwater while trace metals (Al, Fe, Cu, Zn, Pb) were analysed in both soil and extracted soil solution. Concentrations of major ions were high in all samples indicating possible anthropogenic origin. Mean % gravel, % sand, % clay, bulk density, volumetric water content and porosity were 28.8, 63.93, 6.6, 1 g cm−3, 35 and 62.7 %, respectively. Distribution of trace elements showed Kd variation of Al > Cu > Fe > Pb > Zn in the order of sequential increasing solubility. It was observed that the quality of groundwater is not suitable for drinking.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Water is the cheapest and most universally available raw material next to air. It is very essential to man and all living things. Health, nutrition and food production, for example, all depend on the availability of water in adequate quantities and good quality. The uses of water, whether surface water or groundwater, is based on its unique physical and chemical properties which also determine the quality thereof. Sources of water are also major potential pathways by which humans and all life-forms can be exposed to chemicals and pathogens.
Groundwater, as an excellent source of water supply, provides substantial quantity of potable water to many communities in Ghana. It is a major source of water that should be of concern with regards to recent increasing rate of surface water pollution and population growth in most rural communities in Ghana. According to 2009 Annual Ghana Water and Sanitation Sector Performance Report, more than 50 % of the country’s population is living without access to clean water. Water supply to rural and small towns stands at 57.14 per cent, representing a population of 7.22 million. The figure leaves water supply deficit of 5.43 million in the rural areas while the urban coverage is 59 per cent, again leaving a deficit of some 6.1 million Ghanaians without access to potable water.
The main objective of Ghana’s Vision 2025 for water is to ‘promote an efficient and effective management system and environmentally sound development of all water resources in Ghana’ (Boubacar et al. 2005). To realise this objective and get water management issues right, drainage and land use management must also be of great concern and taken care of (Valipour 2012a, b; Gopal et al. 2015). This is a surer way of protecting water resources, especially groundwater from pollution related to anthropogenic activities such as open solid waste disposal practices, a common phenomenon in Ghana.
Since groundwater quality is inextricably linked to the topography that it underlies, which is mostly vulnerable to anthropogenic activities (Moody 1996), the end point of such activities could lead to the storage of pollutants in the unsaturated zone and further transmission into available groundwater over a period of time. The unsaturated zone, described as the portion between the ground surface and groundwater (Freeze and Cherry 1979), is of key interest because the transmission of pollutants to groundwater occurs through it. Periodically, water is added to this zone by rainfall and through irrigation of crops and the portion that is not returned to the atmosphere by evapotranspiration can move downward towards the water table. Under conditions of plenty of water and fractures, water may percolate through the unsaturated zone fairly quickly and may be a major means of recharge. The unsaturated zone may hence serve as a sink for contaminants and transmit pollutants which may have adverse impacts on groundwater quality.
A major source of pollution and contamination of groundwater apart from industrial and agricultural activities is leachate from landfills and waste disposal sites, especially if there is little or no monitoring of who dumps what into it, a situation akin to the Abloradjei solid waste disposal site. Open dump sites also represent a significant source of chemical constituents that may be transmitted from leachate into groundwater (Bretzel and Calderisi 2011; Rizo et al. 2012).
Understanding the behaviour of major ions and trace elements in the unsaturated zone is a major important tool that can be employed in the prediction of pollutant migration, transmission and implications for groundwater, especially in growing cities and communities such as Abloradjei which struggle with problems of environmental contamination, waste storage, land use and water supply. Quantifying the ions and elements in soil and water will also provide valid information of the potential hazards they are likely to pose to the environment and living organisms in events where they exceed thresholds. The occurrence and geographical distribution of certain diseases could be correlated with the presence of toxic elements in the geologic environment as input from anthropogenic sources (Fordyce et al. 2000; Siegel 2002).
It has, therefore, become imperative to critically examine the unsaturated zone as a means of evaluating the behaviour and impacts of leachate contaminants on unsaturated zone water and groundwater quality within the vicinity of the Abloradjei waste disposal site.
The objective of this study was to assess the effect of leachate migration from the Abloradjei waste disposal site on water in the unsaturated zone and the implications for groundwater. The findings will go a long way to help residents make informed decisions regarding their future groundwater quality.
Materials and methods
Study area
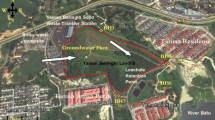
The study area is the Abokobi Municipal solid waste (MSW) disposal site located at Abloradjei in the Ga East Municipal assembly (Fig. 1). The waste disposal site is located on latitude 05°70.494°N and longitudes 0°.19.890°W and it is about 63 m above sea level. The site which covers about ten (10) acres is situated in the middle of settlements with a characteristic wetland nature and has been in operation for the past two decades. A total of 357 tons of solid waste generated daily by the municipal assembly is composed of organic, plastic, metals parts of electronics, bottles/glasses, construction debris, paper and special waste from hospitals. About 63 % of these, in addition to those from neighbouring district and municipal assemblies (Table 1), are collected daily and dumped at the site which is already full.
The community where the site is located has experienced massive increase in population with no access to pipe-borne water and the residents depend heavily on shallow unconfined groundwater between 2 and 8 m and average depth of 5 m as the main source of water supply. Contrary to State-of-the-Art waste disposal practices, the MSW disposal site is not properly sited. There is no segregation of waste at source, and there is no specific disposal site for each type of waste. Also, pre-treatment is not carried out before disposal. The waste piles are not covered; there are no bottom liners, and no leachate treatments. Monitoring wells to serve as check for possible groundwater pollution are also unavailable. Scavengers always set fires openly on site to the waste in search of retrievable materials, a situation which will normally results in thick fumes and ash formation, dissolution and migration of chloride compounds and other pollutants into nearby surface water. Worst of all, great portions of the waste disposal site are being reclaimed for human settlements and other social amenities such schools and churches.
There are few rivers and seasonal streams most of which are threatened by human activities. Groundwater quality, therefore, could not be but an alternative option of water for communities in these rural settlements.
The dominant vegetative zones in the study area are shrubs and grasslands. The shrub lands occur mostly in the western outskirts and in the north towards the Aburi hills and consist of dense cluster of small trees and shrubs that grow to an average height of about five metres. Rainfall is bi-modal with annual temperature ranging between 25.1 °C in July/August and 28.4 °C in February/March. Mean relative humidity is high within a 24-hour period with lowest relative humility in January and highest in August.
Geology
The area is the boundary between two bedrock formations in the Precambrian Guinea Shield of West Africa, namely the metamorphosed and folded Dahomean system and the Togo series (Kesse 1985, as cited in Kortatsi et al. 2008; Fianko et al. 2009). It is located along the Akwapim fault zone (Akoto and Anum 1992) and has been subjected to intense directional metamorphism and pressure in a NW–SE direction resulting in intense folding, fracturing and faulting in phyllite and quartzzites. It consists of gentle slopes interspersed with plains in the west and generally underlain mainly by rocks of Dahomeyean: muscovitebiotitic gneiss, quartz-feldspar gneiss, augen gneiss and minor amphibolites and the Togo series that contain metamorphosed sedimentary rocks such as quartz, schist and phyllites which are susceptible to fracturing upon stress. The area has well-drained, red sandy clay loam to clay with abundant ironstone concretions and gravels. The lithological details are presented in Fig. 1.
Methodology
Fieldwork
To have a prior knowledge of the type of contaminants that may be present in the leachate, waste characterization was done based on existing data at the Abokobi Municipal Health Office and Waste Management Unit (A.M.H.O and W.M.U) as presented in Table 1. A total of three leachate samples, two hand-dug wells, twenty-five soil samples and six unsaturated zone water samples from installed piezometers were taken in November, 2010 and January, 2011, respectively. Leachates were sampled along leachate streams based on the direction of flow, approximately at 100 m intervals from the waste disposal site towards residential areas to identify likely trend in concentration of analysed parameters.
All the water samples were collected in 500 ml pre-conditioned high-density polyethylene bottles. They were conditioned by washing initially with detergent, then with ten per cent (10 %) nitric acid and finally rinsing several times with distilled water. This was carried out to ensure that the bottles were cleaned to avoid contamination.
A hand held auger was used to sample about 500 g fresh soils at 0.5 m depth intervals until water was reached within the unsaturated zone which was the main focus of interest in this study. The soil strata of the study area were classified in situ using Munsell soil colour chart and texture and wetness determined after Faniran and Areala (1978). The soil samples were collected using plastic trowel into clean polyethylene bags (Dampare et al. 2005; IAEA Tec Doc 2009; TVA 2010; Bam et al. 2011a, b). The bags were immediately zipped up at the mouth, labelled well and kept in ice-chests at a very low temperature to protect the samples from physical and chemical changes such as drying and oxidation as much as possible.
Slots of two-inch P.V.C pipes with end caps (piezometers) were inserted into the drills and installed as wells for sampling for physical and hydrochemical analyses to determine the mechanism and extent of leachate migration. The slots were cut liberally vertical to allow percolation of water, but the cuts were small enough to exclude significant intake of soil.
Water from the peizometers was pumped using a peristaltic pump 12VDC with tube specification of min. 2.0 and max. 2.2. At each sampling site, the polyethylene sampling bottles were rinsed at least three times with the sample before sampling was done. About 100 ml of the samples was collected in a conical flask for field analyses. pH, temperature, EC, TDS, salinity and alkalinity were determined in situ. A HACH potable meter was used to measure pH and temperature, while EC and TDS were also measured using the conductivity meter. Both the pH and conductivity meters were placed simultaneously into the beaker containing water sample and the readings taken each time the meter stabilized. The pH meter was calibrated with standard pH buffers 4 and 7 and the conductivity meter was also calibrated with a 2191 µS/cm standard.
Laboratory work and analysis
Various methods for soil water estimation exist (Richards 1954, Day 1965 as cited in Suresh et al. 2008). Soil water content was obtained by dividing the differences of the masses of the wet and dry soils (oven dry at 105 °C for 48 h) by the mass of the dry soil sample from which the percentage water content was also obtained by multiplying the ratio by 100 (Jury et al. 1991). Gravimetric (Θg) and (Θv) volumetric water contents were then obtained as
Also, Θv = Θg × BD, where BD is the bulk density of the soil sample.
The corresponding porosities were also determined as
The various soil particle sizes were also determined by passing known weights of homogenised samples through a set of sieves of known mesh sizes: 2.360, 1.180, 0.280, 0.075 and 0.045 mm, respectively, with a pan below. The sieves were arranged in downward decreasing mesh diameters and mechanically vibrated on a shaker (RETSCH) for a fixed period of 10 min for each sample analysed. After each complete period, the sieves and pan were cleaned with acetone using cotton wool to avoid cross contamination of samples. While the sieves were being shaken, each zip lock polyethylene container for transfer of soil sample from each sieve was weighed to 0.1 gramme. These have been labelled earlier corresponding to each sieve size. The weight of soil retained on each sieve was measured and converted into a percentage particle size of the total soil sample:
where W Sieve is the weight of aggregate in the sieve and W total is the total weight of the aggregate.
For error-free evaluation, the percentage loss of sand was calculated for each measured sample which was far less than the recommended maximum 2 % in each case by protocol (Advantec and ASTM 2001; Mamlouk and Zaniewski 1999).
Aliquots of 8.0 g each of the dried, homogenised soil samples (45 μm) weremixed with 100.0 ml de-ionised water (0.79–4 μS/cm) in the ratio (R) 2:25 in pre-conditioned Teflon containers and leached (Di bonito 2005). The mixture was then agitated vigoroThe ratio of metal concentrationusly by mechanical shaker to ensure mechanical mixing for 6 h and then allowed to settle for an hour.
The supernatant was collected and the pH, Eh, temperature, conductivity and TDS of the leached soils were determined. Each supernatant was also centrifuged in an IEC Centra GP8R refrigerated centrifuge model at 3500 rpm for 15 min. The eluate was then filtered through a 0.45-μm cellulose acetate membrane filter (Toyo Roshi Kaisha, Ltd., Japan). Assuming equilibrium between the solid and the aqueous phase was reached by end of the extraction, the distribution coefficient (KD) of major and trace elements can be determined. This relates the extractable fraction to the total concentration in the soil matrix (Sauve et al. 2003; Bradl 2004).
Sodium (Na+) and potassium (K+) were analysed using flame photometer (Sherwood Model 420) while Ca, Mg, Al, Cr, Mn, Fe, Co, Cu, Zn, Cd and Pb were also analysed using Varian AA240FS Fast Sequential Atomic Absorption Spectrophotometer in an acetylene-air flame.
Chloride (Cl−), sulphate (SO4 2−) nitrate (NO3 −) and phosphate (PO4 2−) were analysed using ICS-90 ion chromatography at the Nuclear Chemistry and Environmental Research Centre, Ghana Atomic Energy Commission.
Results and discussion
Physical parameters
Leachate, leached soil water and groundwater samples at the study site were used to make comparison of distribution of ions and trace elements in unsaturated zone and groundwater.
Temperature values for unsaturated zone water and groundwater range from 29.1 to 38.80 °C. The high temperature for the unsaturated zone water (38.80 °C) may favour dissolution of metals. The pH values vary from 6.98 to 8.78 for leachate, which may be due to the fact that the landfill is at a stage where bicarbonates, hydroxides and ammonia are being produced to neutralise low acidic conditions. Unsaturated zone water and groundwater have pH range of 5.04–6.91 indicating relatively mild acidic conditions due to oxidation processes occurring within the unsaturated zone.
EC for leachate, unsaturated zone water and groundwater ranged from 5930 to 14,750 mg/L, 975 to 16,640 μS/cm, and 195 to 2920 μS/cm, respectively. The high EC in leachate compared to that of the other samples may be mainly due to the presence of more dissolved ions from decomposed organic and inorganic salt deposits migrating along with leachate flow from the waste stream from the disposal site. Comparatively high ECs in unsaturated zone water seem to suggest a certain degree of infiltration of leachate along with water through the unsaturated zone toward groundwater. Samples taken in November recorded higher concentrations compared to those taken in January. This could be explained in terms of higher solute dissolution as the month of November 2010 experienced appreciable amounts of rainfall about a week prior to the sampling compared to January 2011. The monthly distribution of rainfall for the years of sampling is presented in Fig. 2 with no rainfall in January, February, March and December, 2011, respectively.
EC values varied inconsistently for piezometer sampling points with distance from waste disposal site suggesting different possible sources and flow pathways (Fig. 3). EC was also relatively high with depth; an observation that could be attributed to contaminant transport and dissolution with increasing depth.
The highest value (16,640 μS/cm), for piezometer sampling AB4 could be attributed to contaminant transport and dissolution with increasing depth. This is because this sampling point is the deepest sampling profile (1.57 m) compared to the others. A significant decrease in EC for leached soil profiles was observed at certain points with increasing depth contrary to others with reverse pattern (Fig. 4). This could be due different rates of solute movement through the soil strata characterized by the complexity of unsaturated zone transport, which is unpredictably influenced by some degree of leaching and dissolution below certain depths at certain periods.
The EC values for unsaturated water and groundwater samples discussed indicate that there is pollution due to human and land use activities. The groundwater cannot, therefore, be recommended for drinking and other domestic purposes since it is difficult to predict the rate of contaminant flow from leachate which is influenced by numerous factors at different periods.
Chemical parameters for leachate and water samples
Human activities such as waste disposal, irrigation and mining could have a significant influence on water quality which is normally shown in the concentrations of dissolved ions in solution. Statistical summary of major ions determined is presented in Table 2.
Sodium and potassium concentrations ranged from 1171 to 3040 mg/L and 1052 to 4720 mg/L, respectively, for leachate. The highest concentration of sodium observed in the leachate might be due to greater decomposition and dissolution of waste food substances and other compounds containing the alkali metal.
Concentration of Na in unsaturated zone water ranges from 203 to 3900 mg/L with mean 1636.70 mg/L while that of K ranges from 18.10 to 780 mg/L with a mean of 350.16 mg/L. It also ranges from 197 to 578 mg/L and average 386.25 mg/L for groundwater. Potassium concentration for groundwater varies from 12.5 to 261 mg/L with a mean of 126.6 mg/L. The concentrations of both elements are higher in leachate compared to unsaturated zone water and groundwater.
Comparing the average concentration of Na (2333.67 mg/L) in unsaturated zone water to that of groundwater (386.25 mg/L) indicates reduction in concentration which can be attributed to attenuation of the ion as it is retained by adsorption on mineral surfaces. Lower values of potassium occurred in unsaturated zone water and groundwater possibly due to high resistance of K minerals normally to weathering.
The fact that the averages of these elements in groundwater exceed by orders of magnitude the standards for drinking water, Na = 200 mg/L; K = 200 mg/L (WHO 2004), means the water is not suitable for domestic purposes.
Leachate (L) samples have average calcium concentration of 234.98 mg/L and that of magnesium is 517.4 mg/L. The mean Ca concentration for unsaturated zone water is 59.7 mg/L and that of Mg is 81.6 mg/L. Groundwater values are Ca: 156.7 mg/L, Mg: 242.4 mg/L. This is an indication that construction wastes containing calcium and magnesium substances are being disposed along with MSW.
The relatively low presence of Ca compared to Mg could be attributed to increasing sulphate activity taking place as CaSO4 is a very stable molecule and sparingly soluble in water.
Chloride values obtained in this study ranged from 2343.55 to 3309 mg/L for leachate, 189.3 to 3799.4 mg/L for unsaturated zone water and 111.2 to 1128.10 mg/L for groundwater. The high concentration of chlorides in leachate samples may be due to degradation into ashes and dissolution of chloride-containing compounds from anthropogenic sources such as of plastics dumped and burnt on the site, fertilisers as well as cementing materials, textiles, paper, pesticide residues, organic waste from humans, animals and plant edibles and other industrial applications. These sources can result in significant concentrations of chloride in groundwater.
Any low level of the ion observed in unsaturated zone water and groundwater may be due to dilution rather than removal since chlorides are usually not attenuated in soil by processes that remove other ions and are extremely mobile under all conditions. A change in chloride concentration is commonly used to assess the variation of leachate dilution (Bilgili et al. 2007).
The fact that chloride levels were averagely high in analysed samples could be attributed to leachate migration; an indication of possible pollution.
A correlation of 0.74 in leachate, 0.97 in unsaturated zone water and 0.84 in groundwater, respectively, for sodium and chloride suggests both ions are from the same source but may be following different flow paths (Fig. 5).
Most of the points shifting towards chloride axis for analysed samples indicate that substances containing chloride are being disposed of at the site and may result in a deeper migration below the unsaturated zone towards groundwater due to its conservative nature. This implies that contaminants in leachate may equally migrate to greater depths into groundwater leading to bad quality of water.
Sulphate concentrations ranged from 24.24 to 47.9 mg/L, 14.24 to 647.57 mg/L and 10.31 to 520.41 mg/L for leachate, unsaturated zone water and groundwater, respectively. The low concentration of sulphate in leachate could be a result of the reducing and anaerobic conditions taking place in the waste due to bacterial decay of organic substances where the sulphates are been converted to sulphides as iron sulphide (FeS) hydrogen sulphide (H2S) or hydrosulphide.
Since sulphide is to be of concern when leachate is at high pH (normally >9), it can be deduced that the disposal site is at initial stage of reducing conditions as it is still receiving waste.
High sulphate content in unsaturated water and groundwater compared to leachate could be attributed to oxidation processes occurring within the unsaturated zone or vertical mass transmission of contaminants.
According to Chapman (1996), sulphate concentrations in natural waters usually range between 2 and 80 mg/L, although they may exceed 1000 mg/L near industrial discharges. High concentrations greater than 400 mg/L may make water unpalatable. The mean sulphate concentrations for unsaturated zone water and groundwater (183, 229.4 mg/L) are below WHO (1996) recommended value of 250 mg/L.
The presence of nitrogen as nitrate (NO3), nitrite (NO2) or ammonia (NH4+) in water gives a general indication of the nutrient status and level of organic pollution.
Nitrate concentration varied from 3.4 to 8.27 mg/L for leachate, <0.001(instrument detection limit) to 278.48 mg/L for unsaturated zone water, <0.001 to 142 mg/L and mean 39 mg/L for groundwater. Leachate has a lower concentration of nitrate compared to unsaturated zone water and groundwater. The elevated nitrate concentration in groundwater could be due to nitrification and leaching below the unsaturated zone water.
Nitrate concentration was observed to increase distance away from the waste disposal site. This is an indication of migration of the anion normally at distances away from input areas, a trend also observed by Hem (1992). This behaviour therefore makes nitrate a conservative tracer.
The source of nitrate in the samples could be due to nitrogenous waste substances such as fertilisers and organic substances disposed at the site, and not agricultural runoff, since there are no major agricultural activities such as arable cropping going on around the area. The mean concentrations of nitrate in water exceed WHO (2004) permissible limit of 10 mg/L and could result in blue baby syndrome (methemoglobinaemia) if used for drinking and other domestic purposes (Appelo and Postma 2005).
The concentration of phosphate varied from below instrument detection limit (<0.001) to 8.37 mg/L, <0.001 to 29.78 mg/L and <0.001 to 169.79 mg/L for in leachate, unsaturated zone water and groundwater. Concentrations in leachate are low compared to groundwater and unsaturated zone water. The high phosphate concentration in unsaturated zone water and groundwater may also be related to vertical migration of leachate and leaching below the unsaturated zone profile since phosphates are not very mobile in soil or sediments (Hem 1992).
The main source could be attributed to domestic and industrial waste, particularly those containing detergents, pesticide residues and phosphate fertiliser compounds or residues being disposed at the Abloradjei dump site. The water, therefore, is not ideal for domestic or agricultural purposes since this may lead to sorption and microbial load in plants, and bioaccumulation in humans, especially through vegetable consumption.
Also, since the rate of formation and inflow of leachate is dependent on a number of variables such as rainfall, ions with lower concentrations in groundwater samples could be elevated due to possible intermittent higher inflows which can render its use unsafe.
Unsaturated zone soil strata properties
Four representative profiles (AB5, AB6, AB7, and AB8) were selected out of the total eight sampling profiles The vertical section of the core obtained and the lithological compositions are shown in Fig. 6 (After Bam et al. 2011a, b). The entire profiles for the study area show similar trends in soil physical properties (Fig. 7). The unsaturated zone soil texture could be described as being homogeneous throughout the entire sampling profiles (Table 3).
The statistical summary of the soil physical properties is also presented in Table 4.
Gravel content generally increased with increasing depth compared to decreasing sand and clay content with depth. The trend of increasing gravel content with depth suggests loosely packed soil aggregates with greater porosity and comparatively lower bulk density. If similar trend continues beyond the unsaturated zone, there could be greater migration of dissolved pollutants from the leachate along with hydraulic gradient towards groundwater system, unless other chemical processes such as adsorption, ion exchange and complexations with less mobile compounds occur.
Decreasing clay content with increasing depth indicates high probability of contaminant transport from leachate from the Abloradjei waste disposal site through the unsaturated zone to groundwater.
Bulk density decreased with decreasing soil and clay content with increasing depth and inversely with porosity. In a similar study carried out on depth profiles in the Densu River basin Adomako et al. (2010) indicated that porosities ranged from 34 % near surface soil to 41 % at 5.5 m. Thus, both bulk density and porosity are major factors that control hydraulic conductivity. For most soils, bulk densities will be found in a range of 1 to 2 g cm−3, with particle density being 2.652 g cm−3 (Grossman and Reinsch 2002).
Greater porosity with increasing depth will result in higher hydraulic conductivity which describes the degree of fluid flow through a medium and will also allow for greater mechanical diffusion as a function of continuous migration of contaminants through the unsaturated zone towards groundwater.
A general trend of increasing volumetric water content with increasing depth within the entire sampling profiles in the unsaturated zone was also observed. Greater water content can be attributed to greater infiltration rate during sampling. This could also be as a result of continuous wetness due to water inflow from natural water courses or streams resulting in higher zone saturation. Higher water content within the unsaturated zone will result in greater solvation and dissolution of solute contaminants and their transmission towards groundwater. Water content can also be used to predict and possibly determine the fate of trace elements and nutrients (Borggaard and Elberling, 2004).
The mean soil particle size and property distribution for the entire area is 65.60 % ± 0.7 for sand, 29.12 % ± 0.7 for gravel and 6.57 % ± 0.7 for clay, 1.0 g cm3− for bulk density, 35 % for porosity and 63 % for volumetric water content (Fig. 8).
Physicochemical properties of leached soils
Soil pH, Eh and electrical conductivity (EC) from leached soil solution were used to further predict the degree of dissolution and migration trend (leaching) of soil nutrients and elements within the unsaturated zone towards groundwater (Table 5).
Minimum pH of 4.99 occurred between 0.5 m and below 1.6 m and maximum 6.79 at 0.5 m with average 6.1 pH units indicating moderate acidic nature of the soil solution with increasing depth.
Measured Eh averaged 20.64 mV with minimum -029 at 0.5 m depth and maximum 78 mV to beyond 0.5 m. More positive Eh values occurred within the entire profiles (Fig. 9).
The soil can hence be classified as acidic and moderately oxidizing since strongly oxidizing redox is greater than +100 mV (Taylor and Allen 2006). It was found from this study that the stated redox conditions favoured dissolution of certain metals and also resulted in comparatively high nitrate (NO3 −) and sulphate (SO4 2−) concentrations in the leached soil solutions compared to a similar report by Christensen et al. (1992) who indicated that such conditions result in greater dissolution of metals, and high concentrations of manganese (MnO2), iron as [Fe(OH)3], nitrates (NO3 −) and sulphates (SO4 2−).
The analysed leached water solutions for the entire area have minimum EC of 88.5 μS/cm at 0.5 m and below, maximum 962 μS/cm measured between 0 and 0.25 m and average 269 μS/cm indicating decreasing EC with increasing depth. High EC observed at sampling point AB4 further points to greater extent of vertical leachate migration along with hydraulic gradient.
A gradual decrease in sodium concentrations along the profiles was observed; which might not be an indication of its reduction but rather further leaching of the metal below the unsaturated zone since higher concentrations (1636.7 and 386 mg/L) were detected in both unsaturated zone water and groundwater, respectively. Similar trend was observed for potassium with maximum concentration 112.8 mg/L, minimum 3.9 mg/L, (Fig. 10), compared to unsaturated zone and groundwater concentrations (350 and 126 mg/L).
Sulphate concentration ranged from below detection at 0.5 m depth to 1060.9 mg/L at 0.25 m and with average 293 mg/L. Concentrations vary inconsistently with profiles. It was observed that very high values (828, 619, 365 and 952 mg/L) were measured at greater depths in some profiles and also at the surface for other profiles. Comparing the mean unsaturated zone water and mean groundwater values (229 and 183 mg/L) indicates leaching of considerable amount of the ion into sampled groundwater. The high sulphate content could be attributed to leaching of sodium sulphate compounds such as sulphate fertilisers, household detergents, tanneries products and waste paper materials dumped at the site. Drinking groundwater with elevated levels of sulphate can result in diarrhoea and dehydration (Esteban et al. 1997). Infants are often more sensitive to and at risk of sulphate than adults due to organs development and immunity.
Nitrate has maximum concentrations for example, 13.1, 12.25 and 14.58 mg/L occurring within deeper zones. The average NO3 concentration measured for the entire sampling profiles is 7.43 mg/L. Nitrate concentration for leached soil solution was hence fairly stable with increasing depth. Unsaturated zone water and water groundwater concentrations of NO3 (39 and 60.5 mg/l) were higher in nitrate content compared to that of leached soil (4.95–12.09 mg/L).
Phosphate concentrations for the entire area ranged from below detection limit (<0.001 mg/L) to 18.92 mg/L with average 3.9 mg/L. Most concentrations of phosphate in profiles were in the range of between 10 and 19 mg/L in soil profiles near the waste disposal site irrespective of depth compared to measured values (7.4 and 42.65 mg/L) in unsaturated zone water and groundwater.
Sulphate is the dominance anion among the analysed ions with major amounts of sodium. The relative ion dominance for the leached soil water is in the order SO4 > Na > K > NO3 > PO4 (Fig. 11).
Generally, higher concentrations of the ions occurred in soils sampled at greater depths. Concentrations of major ions analysed in leached soil solution obtained from the entire sampling points were comparatively lower but higher in groundwater and unsaturated zone water. This is indicative of some degree of mobility of the ions towards groundwater, an indication that shallow wells mostly common to the Abloradjei community could be at risk of pollution from leachate and other soil surface activities.
Trace element distribution coefficient (KD) in unsaturated zone profile
The potential risk of heavy metals in soils, with respect to their mobility and ecotoxicological significance, is determined by their solid–solution partitioning (sorption–desorption) rather than the total metal concentration. Solid–solution partitioning is, therefore, critical to assess mobility in soils and the potential of leaching of metals from soil to groundwater.
The ratio of metal concentration in soil-to-metal concentration in soil water (Kd) estimates solubility as
where KD is soil/water distribution coefficient (L kg−1); [Metal] soil = total metal concentration in the fine earth (mg kg−1); and [Metal] water extract = total metal concentration in the water extract (mg L−1). From the above relation, higher KD means less susceptibility of a particular metal to groundwater due to greater adsorption unto the soil.
KD for Al increased with depth from 8.06 at 0.5 m to 298.13 at 1.2 m, a trend which compares positively with the total metal concentration in soil but with reduction in water samples.
The mean KD for Fe: 13.4 compared with the mean total concentration of 173 mg/Kg indicates almost 13 times (8 %) attenuation of the metal before reaching groundwater.
Cu has maximum KD 43 and minimum 1.8 both at 0.5 m depth and mean 39.5. This indicates the total removal of Cu by underlying soil, a further confirmation of its concentration <0.003 detection limit in all water samples. Zn has KD range of 2.04 at 0.25 m depth to 6.5 at 1.5 m with mean 4.03, indicating a fairly stable KD with slight increase with depth.
KD for Pb ranged from <0.002 closer to the soil surface to maximum of 33.30 below 0.5 m which occurred in samples closer to the waste disposal site, showing an increasing trend with depth in Pb concentration. This accounts for the mean concentration of the element (0.02 mg/L) slightly higher than WHO recommended drinking water level (0.01 mg/L).
The order of KD values is Al > Cu > Fe > Pb > Zn, indicating a sequential increasing solubility. Groundwater in the area could, therefore, be at risk of Pb contamination.
An empirical linear relationship between KD and soil properties: pH and total metal content were also employed to establish which component contributed most in solubility of the trace elements.
The linear correlation between KD and soil pH solution ranged between 1.2 and 7 % for the variation of Fe, Al, Cu, Pb and Zn, respectively. More so, the linear relationship between KD and total metal concentrations gave the variation 4 % Al, 8.4 %, 26 % Fe Cu, 59 % Zn and 73 % Pb (Figs. 12, 13).
It could, therefore, be established from this study that the degree of effective leaching of the heavy metals from the soil surface to the groundwater system is more controlled by the total metal content [M] rather than pH; an indication of land use activities which could be mainly due to waste disposal resulting in the release and leaching of the trace elements into solution since no other activities such as farming are ongoing around the area of study.
Conclusion
High ECs were recorded for unsaturated zone and groundwater samples. There is evidence of leaching of major ions below the sampled depths of the unsaturated zone in the vicinity of the Abloradjei waste disposal site. The high dissolved solid content in shallow groundwater in the area renders it unsuitable for domestic and irrigation purposes. The water may also be a source of microbial load if used for vegetables.
Percentage gravel and volumetric water contents increased with increasing depth compared to decreasing sand and clay contents. This suggests loosely packed soil aggregates with higher porosity which predicts greater transmission of dissolved pollutants from leachate along with hydraulic gradient toward groundwater unless chemical and natural processes of retardation occur.
Sulphate is the dominant anion among the analysed ions with major amounts of sodium for leached soil. Generally, higher concentrations of the ions occurred in soils sampled at greater depths. Concentrations of the major ions in leached soil solution were comparatively lower but higher in unsaturated zone water and groundwater, an indication of migration below sampled depths within the unsaturated zone. Shallow wells and groundwater as mostly common to the Abloradjei area could, therefore, be at risk of pollution from leachate.
The order of distribution coefficient of sorption/desorption between the soil and water equilibrium phase is Al > Cu > Fe > Pb > Zn, indicating a sequential increasing solubility. Empirical linear relationship between KD versus soil pH and total metal concentration showed that total metal content [M] controlled more of the dissolved metals in soil solution rather than pH.
However, due to variable factors controlling the formation and migration of leachate, chief of which is rainfall, coupled with the complex nature of the unsaturated zone, fluctuation in the concentrations of measured parameters can be expected sporadically.
References
Adomako D, Maloszewski P, Stumpp C, Osae S (2010) Estimating groundwater recharge from water isotope (δ2H, δ18O) depth profiles in the Densu River Basin. Hydrol Sci J 55(8):1405–1416
Akoto MA, Anum SA (1992) Monitoring Recent Microseismic Activity in Ghana
Appelo CAJ, Postma D (2005) Geochemistry,Groundwater and Pollution, 2nd edn. Balkema Publishers, Rotterdam
ASTM (2001) Advantech Manufacturing and Inc., New Berlin
Bam EKP, Akiti TT, Osae S, Ganyaglo SY, Adomako D, Gibrilla A. Abass, Godfred A (2011a) Major Ions and Trace elements Partitioning in Unsaturated Zone Profile of the Densu River Basin, Ghana and the Implications for groundwater. Afr J Environ Sci Technol 5(6):427–436. www.academicjournals.org/ajest
Bam EKP, Akiti TT, Osae S, Ganyaglo SY, Adomako D, Gibrilla A, Abass, Godfred A (2011b) Multivariate cluster analysis of some major trace elements distribution in. Afr J Environ Sci Technol 5(3):155–167. www.academicjournals.org/ajest
Bilgili MS, Demir A, Bestamin O (2007) Influence of leachate recirculation on aerobic and anaerobic decomposition of solid wastes. J Hazard Mater 143:177–183
Borggaard OK, Elberling BO (2004) Pedological Biogeochemistry, Institute of Geography Denmark, University of Copenhagen, p 488
Boubacar B, Emmanuel O, Marc A, Winston A and Mathilde P (2005) Comprehensive assessment of water management in agriculture comparative study of river basin development and management, International Water Management Institute
Bradl HB (2004) Adsorption of heavy metal ions on soils and soils constituents. J Colloid Interface Sci 277:1–18
Bretzel FC, Calderisi M (2011) Contribution of a municipal solid waste incinerator to the trace metals in the surrounding soil. Environ Monit Assess 182:523–533
Chapman (1996) Water quality assessments—A guide to use of biota, sediments and water in environmental monitoring, 2nd edn. Great Britain at the University Press, Cambridge, pp 74–112
Christensen TH, Raffaello C, Rainer S (1992) Landfill Leachate. In: Land Filling of Waste Leachate, St. Edmundsbury Press, Bury St
Dampare SB, Ameyaw Y, Adotey DK, Osae S, Serfor-Armah Y, Nyarko BJB, Adomako D (2005) Seasonal trend of potentially toxic trace elements in soils supporting medicinal plants in the eastern region of Ghana, Springer. Water Air Soil Pollut 169:185–206
Di Bonito M (2005) Trace elements in soil pore water: a comparison of sampling methods, University of Nottingham
Faniran A, Areola O (1978) Essentials of Soil Study with Special Reference to Tropical Areas Heinemann. UK, London
Fianko JR, Nartey VK, Donkor A (2009) The hydrochemistry of groundwater in the Densu River Basin, Ghana, Environ. Monit. Assess., Springer Science + Business Media B.V
Fordyce FM, Guangdi Z, Green K, Xinping L (2000) Soil, grain and water chemistry in relation to human selenium- responsive diseases in Enshi district, China. Appl Geochem 15:117–132
Freeze R, Cherry J (1979) Groundwater. Prentice Hall, Edgewood Cliffs
Gopal K, Lohani AK, Rao MS, Kumar S, Takshi KS (2015) Spatiotemporal variability analysis of groundwater level for water resources development and management in Northern Punjab, India
Grossman R.B, Reinsch T.G. (2002) SSSA Book Series: 5 Methods of Soil Analysis Ch2, Ed
Hem JD (1992) Study and Interpretation of the chemical characteristics of natural waters. Water Supply Paper, 2254, 3rd edn, US Geological Survey. Washington DC, p 263
IAEA-TECDOC-1618 (2009) Application of isotopes to the assessment of pollutant behaviour in the unsaturated zone for groundwater protection, IAEA, Vienna
Jury W, Gardner WR, Gardner WH (1991) Soil Physics. Academic Press, New York
Kortatsi BK, Asigbe J, Dartey DA, Tay C, Anornu GK, Hayford E (2008) Reconnaissance survey of arsenic concentration in ground-water in south-eastern Ghana, West African Journal of Applied Ecology, vol. 13, Ecological Laboratory, University of Ghana, Legon
Kumar S, Krishan G, Saha SK (2008) Measuring Soil Salinity with WET Sensor and Characterization of Salt Affected Soils Agropedology, 18 (2):124–128
Mamlouk MS, Zaniewski JP (1999) Materials for Civil and Construction Engineers, Addison-Wesley, Menlo Park CA Natural Water, 3rd edn. US Geological Survey Water-Supply, Washington
Moody DW (1996) US geological survey, sources and extent of groundwater contamination
Rizo OD, Merlo MH, Castillo FE, Lopez JAO (2012) Assessment of metal pollution in soils from a former Havana (Cuba) solid waste open dump. Bull Environ Contam Toxicol 88:182–186
Sauve S, Manna S, Turmel M, Roy AG, Courchesne F (2003) Solid—solution partitioning of Cd, Cu, Ni, Pb and Zn in the organic horizons of a forest soil. Environ Sci Technol 37:5191–5196
Siegel FR (2002) Environmental geochemistry of potentially toxic metals. Springer, New York 218
Taylor R, Allen A (2006) Waste disposal and landfill: Potential hazards and information needs, London
TVA (2010) Tennessee Valley Authority Monitoring Well and Piezometer Installation and Completion, TVA- KIF-SOP-39, pp 1–20
Valipour M (2012a) A comparison between horizontal and vertical drainage systems (include pipe drainage, open ditch drainage, and pumped wells) in anisotropic soils. J Mech Civi Eng 4:7–12 (20)
Valipour M (2012b) Effect of drainage parameters change on amount of drain discharge in subsurface drainage systems. J Agric Vet Sci 1:10–18
WHO (1996) World Health Organization guidelines for drinking water quality. Inorganic Constituents and Physical Parameters, vol 2, 2nd edn, p 165
WHO (2004) World Health Organization Guidelines for Drinking-water Quality, 1st Addendum to the 3rd edn. vol. 1, Recommendations, Geneva, pp 296–400. (ISBN 9245 1546387)
Acknowledgments
The authors are indebted to the technicians and technologists of the Nuclear Chemistry and Environmental Research Centre of the National Nuclear Research Institute, Ghana Atomic Energy Commission, for their role in the analyses.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Egbi, C.D., Akiti, T.T., Osae, S. et al. Assessment of groundwater quality by unsaturated zone study due to migration of leachate from Abloradjei waste disposal site, Ghana. Appl Water Sci 7, 845–859 (2017). https://doi.org/10.1007/s13201-015-0297-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13201-015-0297-8