Abstract
Purpose
Excessive supraglottic airway cuff pressure increases postoperative pharyngolaryngeal symptoms such as sore throat, dysphonia, and dysphagia. A new supraglottic airway, AES Ultra CPV™ (CPV), has a built-in intracuff pressure indicator. We hypothesized that using the CPV would reduce postoperative symptoms when compared with the LMA Classic™ (LMA) without intracuff pressure guidance.
Methods
Ambulatory patients undergoing general anesthesia were randomized to either CPV or LMA. A size 3/4/5 was inserted according to manufacturer guidelines. Nitrous oxide was not used. In the LMA Group, the cuff was inflated according to manufacturer’s guidelines. In the CPV Group, a CPV was inserted and the cuff inflated until the indicator was in the green zone (30-44 mmHg). Intracuff pressures were measured at five minutes and 20 min post-insertion in both groups. The primary outcome was the incidence of pharyngolaryngeal symptoms, defined as sore throat, dysphonia, and/or dysphagia at one, two, and/or 24 hr postoperatively. Continuous data were compared using Student’s t test and categorical data were analyzed using Chi square analysis.
Results
The study included 170 patients, 85 per group. The mean (SD) intracuff pressure in the CPV group was significantly lower [44 (4) mmHg] than in the LMA Group [87 (37) mmHg]; P < 0.001. The incidence of pharyngolaryngeal symptoms was significantly lower in the CPV Group than in the LMA Group (26% vs 49%; P = 0.002). The absolute risk reduction was 24%, and the number-needed-to-treat was 4.3.
Conclusion
The incidence of postoperative pharyngolaryngeal symptoms in the CPV Group with a cuff pressure-guided strategy was significantly lower than in the LMA Group with standard practice. (Clinical trial registration number: NCT01800344).
Résumé
Objectif
Une pression excessive du ballonnet d’un dispositif supraglottique augmente les symptômes pharyngo-laryngés postopératoires tels que mal de gorge, dysphonie et dysphagie. Un nouveau dispositif supraglottique, l’AES-Ultra-CPV™ (CPV), comporte un indicateur intégré de pression du ballonnet. Nous avons formulé l’hypothèse que l’utilisation du CPV diminuerait les symptômes postopératoires par rapport au LMA Classic™ (LMA) sans indication de la pression intraballonnet.
Méthodes
Des patients ambulatoires subissant une anesthésie générale ont été randomisés à recevoir un CPV ou un LMA. Un dispositif de taille 3, 4 ou 5 a été inséré conformément aux recommandations du fabricant. Le protoxyde d’azote n’a pas été utilisé. Dans le groupe LMA, le ballonnet a été gonflé conformément aux recommandations du fabricant. Dans le groupe CPV, un CPV a été inséré et le ballonnet a été gonflé jusqu’à ce que l’indicateur soit dans la zone verte (30 à 44 mmHg). Les pressions intraballonnet ont été mesurées à cinq minutes et 20 minutes après l’insertion dans les deux groupes. Le critère d’évaluation principal était l’incidence des symptômes pharyngo-laryngés, définis comme étant un mal de gorge, une dysphonie et/ou une dysphagie, à une, deux et/ou 24 heures postopératoires. Les données continues ont été comparées en utilisant le test t et les données catégorielles ont été analysées par le calcul du Chi-carré.
Résultats
Le nombre total de patients était 170, soit 85 dans chaque groupe. La pression intraballonnet a été significativement plus faible dans le groupe CPV (44 [4] mmHg) que dans le groupe LMA (87 [37] mmHg); P < 0,001. L’incidence des symptômes pharyngo-laryngés était significativement plus basse dans le groupe CPV que dans le groupe LMA (26 % contre 49 %, P = 0,002). La réduction du risque absolu était de 24 % et le nombre de patients à traiter était de 4,3.
Conclusion
L’incidence des symptômes pharyngo-laryngés postopératoires a été significativement plus basse dans le groupe CPV avec une stratégie de gonflage du ballonnet à pression contrôlée par rapport au groupe LMA utilisant les normes habituelles de pratique. (Numéro d’enregistrement de l’essai clinique: NCT01800344).
Similar content being viewed by others
In the United States, the number of outpatient procedures increased from 380,000 in 1983 to 53.3 million in 2006.1 In ambulatory surgery, patient dissatisfaction has been shown to be related to the number of postoperative symptoms.2 A significant factor associated with patient dissatisfaction is the presence of sore throat.2
The LMA Classic™ (LMA) is easy to insert, less invasive than a tracheal tube, and provides a satisfactory ventilatory conduit during general anesthesia.3-6 Thus, many anesthesiologists favour LMAs as the airway device of choice for patients undergoing ambulatory surgery. The incidence of pharyngolaryngeal morbidity is found to be 34-42%.7-10 The LMA, a large foreign body, exerts pressure on the pharyngeal mucosa to create an airway seal. There is speculation that high LMA intracuff pressures reduce mucosal perfusion, leading to sore throats.8 Results of two randomized controlled trials showed that appropriate monitoring and reduction of intracuff pressure resulted in less pharyngolaryngeal symptoms.9,10 In practice, manometers for measuring intracuff pressure are not readily available in the operating room. Also, spot measurements of intracuff pressure using external manometers do not provide continuous assessment during surgery. Therefore, implementation of intracuff pressure measurement and reduction is difficult to achieve in routine practice.
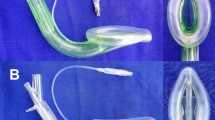
A new supraglottic airway device, the Ultra CPV™ (Cuff Pilot Valve) (AES, Inc., Black Diamond, WA, USA), possesses supraglottic airway features that are virtually identical to the LMA Classic™. The cuffs of both devices are made of silicone, and the shape of the cuff and tube of the two devices are very similar (Fig. 1). There are two main differences between the two airway devices; the CPV does not have aperture bars at the distal end, and it has an integrated cuff pilot balloon valve that provides continuous monitoring of the intracuff pressure. Inflating the pilot balloon to attain the green zone on the indicator translates into a pressure of 30-44 mmHg (Fig. 1). We hypothesized that using the CPV with an intracuff pressure-guided strategy would reduce the incidence of postoperative sore throats, dysphonia, and/or dysphagia when compared with using the LMA with standard practice.
Methods
This randomized controlled trial received approval from the Institutional Research Ethics Board (REB number 11-0392-A) of the University Health Network on December 5th, 2011. A research assistant obtained written informed consent from ambulatory patients undergoing knee arthroscopy, plastic, gynecological, or general surgical procedures of less than two-hour duration under general anesthesia. Inclusion criteria were patients aged 18-65 yr and American Society of Anesthesiologists (ASA) physical status I-III. Patients were excluded if they had a body mass index (BMI) > 40 kg·m−2, a mouth opening < 2.5 cm, symptomatic hiatus hernia, esophageal reflux disease, recent history of upper respiratory tract infection, sore throat, dysphonia, or dysphagia.
On the day of surgery, patients were randomized to receive either a LMA (LMA Classic, Canada Teleflex Medical, Markham, ON) or a CPV (AES Ultra CPV, AES, Inc., Black Diamond, WA) for airway management. An independent statistician randomized the patients according to a computer-generated block randomization scheme (17 blocks, ten participants in each block). The randomization labels were placed in sealed individual envelopes, and the envelopes were opened after receiving consent from the patients. Anesthesiologists with at least one year of experience and having previously inserted 100 supraglottic airways participated in this study. A standardized general anesthetic technique was used. Patients were pre-oxygenated and anesthesia was induced with fentanyl 1-2 μg·kg−1 and propofol 2-3 mg·kg−1. Neuromuscular blocking agents were not used. Bag and mask manual ventilation was instituted with 100% oxygen. A Guedel-type airway was used if manual ventilation was difficult. Subjects weighing 30-50 kg were given a size 3 LMA or CPV; 50-70 kg, size 4; and > 70 kg, size 5, as per the manufacturers’ recommendations. Minor size variation was permitted at the discretion of the attending anesthesiologist.
In the LMA group, the device was completely deflated before insertion. Once the desired depth of anesthesia (jaw relaxed and loss of eyelash reflex) was achieved, a dorsally lubricated LMA was inserted. The LMA cuff was inflated by the attending anesthesiologist according to the manufacturer’s guidelines and at the anesthesiologist’s discretion. The manufacturer recommended an inflating volume of up to 20, 30, and 40 mL of air for size 3, 4, and 5, respectively. The anesthesiologist was permitted to palpate and inflate the pilot balloon cuff at his/her discretion. The intracuff pressure was not measured. Once inserted, ventilation was assessed by examination of capnography and thoracoabdominal movement. If an air leak was detected at 20 cm H2O (14.7 mmHg) positive pressure ventilation, up to two incremental 5-mL intracuff air inflations were permitted until a seal was attained. If the additional inflation was inadequate in attaining a seal, then one reinsertion attempt with the same or different size device was allowed. If there was difficulty inserting the LMA, requiring more than two attempts, the case was excluded. Five to ten minutes post-insertion, the anesthesiologist was asked to grade the insertion as easy, fair, or difficult.
In the CPV group, CPV airway size selection, insertion technique, and anesthetic management were identical to that of the LMA group, except for the inflation method. The CPV cuff was inflated by the attending anesthesiologist, instructed by a research assistant, until the cuff pressure indicator was within the green zone (30-44 mmHg). If an air leak was detected at 20 cm H2O (14.7 mmHg) positive pressure ventilation, an additional 5 mL of intracuff air inflation was permitted, provided that the cuff pressure indicator was not originally in or did not reach the red zone. One reinsertion attempt with the same or different size device was allowed. If there was difficulty inserting the CPV, requiring more than two attempts, the case was excluded. Five to ten minutes post-insertion, the anesthesiologist was asked to grade the insertion as easy, fair, or difficult.
All patients were maintained with an air-oxygen mixture, F i O2 of 0.3-0.5, and desflurane to achieve 0.8-1.4 MAC. Assisted manual ventilation was initiated and spontaneous ventilation was permitted once the patient resumed breathing. Nitrous oxide was not used. Additional doses of fentanyl 25 μg were used at the discretion of the anesthesiologist.
A research assistant measured the intracuff pressure in both groups using an external pressure manometer (Portex® Pressure Gauge, Smith Medical International Ltd, United Kingdom) at five and 20 min after anesthesia induction. The anesthesiologist was blinded to the intracuff pressure measurements and was instructed not to measure or modify intracuff pressure during the surgical procedure.
At the end of surgery, when the patient was awakened and obeying commands, the supraglottic airway was deflated and removed. Pharyngeal suctioning was not performed unless necessary. Patients were transferred to the postanesthesia care unit (PACU). Fentanyl 25-50 μg was administered as needed to treat postoperative pain. A research assistant, blinded to patient allocation, interviewed patients at one and two hours after tracheal extubation to obtain outcome data regarding sore throat, dysphonia, and dysphagia. Patients were discharged home after they fulfilled the post-anesthetic discharge scoring criteria.11 A telephone interview was conducted 24 hr after surgery to obtain outcome data.
The primary outcome was composite pharyngolaryngeal symptoms, defined as sore throat, dysphonia, and/or dysphagia at one, two, and/or 24 hr postoperatively. Sore throat was defined as “constant pain or discomfort in the throat independent of swallowing.” Dysphonia was defined as “difficulty speaking or pain on speaking”, and dysphagia was defined as “difficulty or pain provoked by swallowing.”
A research assistant collected the following preoperative, intraoperative, and postoperative data:
-
Preoperative data: age, ASA physical status, height, weight, BMI, and neck circumference; Intraoperative data: intracuff pressure five and 20 min after induction of anesthesia, duration of general anesthesia, size of supraglottic airway, anesthesiologist’s experience (< five years, five to ten years, > ten years), number of insertion attempts, use of Guedel-type airway for mask ventilation, presence of laryngospasm, total fentanyl usage, presence of blood on supraglottic airway after removal, and use of pharyngeal suctioning;
-
Postoperative data: duration of PACU stay, nausea and vomiting in the PACU, patient satisfaction of the anesthetic technique, and total fentanyl usage in the PACU. Postoperatively at one, two, and 24 hr, patients were asked regarding the presence of sore throat, dysphonia, and/or dysphagia (yes or no). At two hours postoperatively, they were also asked to rate their satisfaction with the anesthetic given (ten-point Likert scale).
Based on the 34-42% incidence of postoperative pharyngolaryngeal symptoms in the literature,7-10 we hypothesized that using a CPV pressure-guided strategy would reduce pharyngolaryngeal symptoms by 50%. To achieve 80% power and an alpha error of 0.05, a sample size of 152 was required, 76 in each group. To account for a dropout rate of 10%, a sample size of 170 patients was used, 85 in each group. Data were analyzed by SPSS® 15.0 (SPSS, Chicago, IL, USA). The primary outcome, composite pharyngolaryngeal symptoms, was analyzed using Chi square analysis. All categorical data were analyzed using Chi square analysis, continuous data were analyzed using Students t test, and nonparametric data were analyzed using Mann-Whitney U tests. P < 0.05 was considered statistically significant. Bonferroni correction was used to account for multiple comparisons.
Results
Patients were screened over a seven-month period (March 1-Oct 1, 2012). One hundred and ninety-nine patients were screened and 175 consented to participate in the study. Five patients were withdrawn prior to surgery due to surgery cancellation. One hundred and seventy patients entered the study, 85 in each group. The patients’ age, sex, BMI, and perioperative variables were similar between the two groups (Table). All insertions were successful by the second attempt in both the CPV and LMA groups.
The mean (SD) intracuff pressure was significantly lower in the CPV group than in the LMA group at five minutes [44 (4) mmHg vs 87 (37) mmHg, respectively; (P < 0.001)] and at 20 min post-insertion [44 (4) mmHg vs 86 (36) mmHg, respectively; (P < 0.001)]. There was no difference in the intracuff pressure between five minutes and 20 min post-insertion in either group. The incidence of pharyngolaryngeal symptoms was significantly lower in the CPV Group than in the LMA Group (26% vs 49%, respectively; P = 0.002). Using the CPV with intracuff pressure-guided strategy was associated with an absolute risk reduction of 24%, relative risk reduction of 48%, and the number-needed-to-treat of 4.3 in preventing pharyngolaryngeal symptoms.
Taken individually, the incidences of sore throat, dysphonia, and dysphagia were all significantly less in the CPV group than in the LMA group (Fig. 2). Patient satisfaction was higher in the CPV group than in the LMA group with the median [interquartile range = IQR] of 9 [8-10] vs 8 [7-9], respectively; P < 0.001.
Incidence of sore throat (top), dysphonia (middle), and dysphagia (bottom) at one, two, and 24 hr postoperatively. Dark bars represent the AES Ultra CPV™ (CPV) group and white bars represent the LMA Classic™ (LMA) group. The numbers on top of bars denote percentage of patients with symptoms. Asterisks denote P < 0.05 between groups
In the LMA group, one patient had a cuff leak after the initial insertion and inflation. The leak was resolved after a further 10 mL inflation of air. In a second patient, the first insertion attempt with a size 5 LMA was unsuccessful, but insertion was successful on the second attempt with a size 4 LMA. In the CPV group, one patient had a persistent cuff leak after initial insertion and inflation to the green zone. The CPV was removed and successfully reinserted a second time without a cuff leak.
Discussion
Our study results showed that the incidence of composite pharyngolaryngeal symptoms was significantly lower in the CPV group with a pressure-guided strategy than in the LMA group using standard practice (26% vs 49%, respectively). Intracuff pressure was lower in the CPV group than in the LMA group (44 mmHg vs 86 mmHg, respectively) at five and 20 min post-insertion. In the medical literature, pressure measurements are expressed either in mmHg or in cm H2O. One mmHg is equivalent to 1.36 cm H2O. Results in the discussion section are expressed in both units depending on the original study.
Results of six studies showed that reducing intracuff pressure in a LMA Classic was associated with a decreased incidence of pharyngolaryngeal symptoms.9,10,12-15 Nott et al. 9 conducted a trial comparing a pressure-limited group with the LMA cuff pressure maintained at a “just-seal” level with a standard practice control group. The pressure-limited group had a lower incidence of postoperative sore throat than the control group (7.0% vs 15.7%, respectively; P < 0.001). Brimacombe et al. 12 randomized 200 patients to an initial intracuff volume that was either low (15-20 mL) or high (20-40 mL). The low volume group experienced less sore throat than the high volume group (20% vs 42%, respectively; P < 0.04) and less dysphagia (1% vs 11%, respectively; P < 0.04). Intracuff pressures were not measured in the above two studies. Jeon et al.13 compared surgical patients who received a pressure-limited “just seal” strategy or standard practice controls. The intracuff pressure was significantly higher in the control group. The pressure-limited group had fewer sore throats than the standard group at one hour postoperatively (23.3% vs 70.0%, respectively; P < 0.05). Burgard et al. 14 also studied patients who received a pressure-limited “just seal” strategy or standard practice controls. The intracuff pressure was significantly higher in the control group. The incidence of sore throats at eight hours postoperatively was 0% in the pressure-limited group compared with 8% in the control group. Seet et al.10 compared a pressure-limited group whereby the intracuff pressure was maintained at 44 mmHg with a control group where the intracuff pressure was simply monitored. The intracuff pressure was significantly lower in the pressure-limited group. The incidence of combined pharyngolaryngeal symptoms postoperatively was significantly lower in the pressure-limited group than in the standard group (13% vs 46%, respectively; P < 0.001). An audit by Wong et al.15 involving 400 children found that none of 111 children with intracuff pressures ≤ 40 cm H2O developed postoperative sore throats, while 45 of 289 children with cuff pressures > 40 cm H2O developed postoperative sore throats.
Two studies found no association between intracuff pressure and pharyngolaryngeal symptoms.16,17 Rieger et al. 16 studied patients with intracuff pressures of 30 or 180 mmHg. The incidence of pharyngolaryngeal symptoms was similar between the groups at eight hours (50% vs 42%) and 24 hr postoperatively (23% vs 32%). Figueredo et al. 17 randomized patients to one of four groups: positive pressure ventilation or spontaneous breathing at 50% or 66% nitrous oxide. The intracuff pressures ranged from 195-210 mmHg, and pharyngolaryngeal symptoms 24 hr postoperatively (24.1-36.6%) were similar amongst all groups.
Our results are consistent with the majority of studies in the literature, showing that reduction of intracuff pressure is associated with decreased pharyngolaryngeal symptoms. Our incidence of pharyngolaryngeal symptoms in the LMA (control) group are similar to findings in other studies,10,12 but are higher9,14 or lower13 than others in the literature. This discrepancy may be explained by the differences in the definition of pharyngolaryngeal symptoms and the time points and duration of symptom assessment. The exact pathophysiologic basis behind pharyngolaryngeal symptoms associated with elevated intracuff pressure has not been elucidated. It has been suggested that the LMA, which exerts pressure on the pharyngeal mucosa, may reduce pharyngeal mucosal perfusion and lead to tissue ischemia and damage.18 High intracuff pressure associated with paralysis of the recurrent laryngeal and hypoglossal nerves has been reported.19-21
In many studies, high intracuff pressures with supraglottic airway usage were often reported, which highlights the need to monitor intracuff pressure for patient safety.10,22,23 Despite this, current standard practices do not incorporate measuring intracuff pressure due to several factors. First, intracuff pressure manometers are not widely available in the operating rooms. Second, even if a manometer is available and used for spot reading, it does not provide continuous measurements. Intracuff pressure can change during the course of surgery, especially with use of nitrous oxide and positional changes.17,23 The CPV, which features a built-in intracuff pressure indicator, is convenient and continuously monitors pressure levels. Our results highlight the effectiveness of the CPV strategy; keeping within the green zone is tightly correlated with an intracuff pressure of 44 mmHg.
Limitations
The purpose of this study was to compare postoperative pharyngolaryngeal symptoms using a CPV cuff pressure-guided strategy with those using the LMA standard practice. It was not intended to compare symptoms between two devices, i.e., a CPV and a LMA inflated to attain the same cuff pressure. Second, our findings were from a single institution randomized controlled trial in an academic teaching setting and may not be generalizable to a wider setting. Third, nitrous oxide was not used. We do not know the effect on intracuff pressure and pharyngolaryngeal symptoms if nitrous oxide were utilized. Nevertheless, as the intracuff pressure is continuously monitored throughout surgery, a rise in intracuff pressure would alert the operator to remove air to re-attain the green zone position, and our conclusion should still be valid. Fourth, intracuff pressure was measured at five and 20 min post-insertion, and the results were virtually identical in value at the two time-points for both groups. The intracuff pressure was not measured at other time points during surgery.
Conclusion
Using a CPV supraglottic airway with an intracuff pressure-guided strategy can limit intracuff pressure to 44 mmHg and reduce postoperative pharyngolaryngeal symptoms by 48% compared with the LMA standard practice. Although LMA intracuff pressure can be monitored and limited using an external manometer, it is difficult to apply routinely due to lack of availability. Therefore, it may be easier to apply a cuff-pressure limitation strategy routinely in patients using a CPV supraglottic airway with a built-in intracuff pressure indicator.
References
Cullen KA, Hall MJ, Golosinskiy A. Ambulatory surgery in the United States, 2006. Natl Health Stat Report 2009; 11: 1-25.
Tong D, Chung F, Wong D. Predictive factors in global and anesthesia satisfaction in ambulatory surgical patients. Anesthesiology 1997; 87: 856-64.
Hernandez MR, Klock PA Jr, Ovassapian A. Evolution of the extraglottic airway: a review of its history, applications, and practical tips for success. Anesth Analg 2012; 114: 349-68.
Benumof JL. Laryngeal mask airway and the ASA difficult airway algorithm. Anesthesiology 1996; 84: 686-99.
Henderson JJ, Popat MT, Latto IP, Pearce AC, Difficult Airway Society. Difficult Airway Society guidelines for management of the unanticipated difficult intubation. Anaesthesia 2004; 59: 675-94.
Brimacombe J. The advantages of the LMA over the tracheal tube or facemask: a meta-analysis. Can J Anaesth 1995; 42: 1017-23.
Rice MJ, Gravenstein NL, Brull SJ, Morey TE, Gravenstein N. Using the inflating syringe as a safety valve to limit laryngeal mask airway cuff pressure. J Clin Monit Comput 2011; 25: 405-10.
O’Kelly SW, Heath KJ, Lawes EG. A study of laryngeal mask inflation. Pressure exerted on the pharynx. Anaesthesia 1993; 48: 1075-8.
Nott MR, Noble PD, Parmar M. Reducing the incidence of sore throat with the laryngeal mask airway. Eur J Anaesth 1998; 15: 153-7.
Seet E, Yousaf F, Gupta S, Subramanyam R, Wong DT, Chung F. Use of manometry for laryngeal mask airway reduces postoperative pharyngolaryngeal adverse events: a prospective, randomized trial. Anesthesiology 2010; 112: 652-7.
Chung F, Chan VW, Ong D. A post-anesthetic discharge scoring system for home readiness after ambulatory surgery. J Clin Anesth 1995; 7: 500-6.
Brimacombe J, Holyoake L, Kellar C, et al. Pharyngolaryngeal, neck, and jaw discomfort after anesthesia with the face mask and laryngeal mask airway at high and low cuff volumes in males and females. Anesthesiology 2000; 93: 26-31.
Jeon YS, Choi JW, Jung HS, et al. Effect of continuous cuff pressure regulator in general anaesthesia with laryngeal mask airway. J Int Med Res 2011; 39: 1900-7.
Burgard G, Mollhoff T, Prien T. The effect of laryngeal mask cuff pressure on postoperative sore throat incidence. J Clin Anesth 1996; 8: 198-201.
Wong JG, Heaney M, Chambers NA, Erb TO, von Ungern-Sternberg BS. Impact of laryngeal mask airway cuff pressures on the incidence of sore throat in children. Pediatr Anesth 2009; 19: 464-9.
Rieger A, Brunne B, Striebel HW. Intracuff pressures do not predict laryngopharyngeal discomfort after use of the laryngeal mask airway. Anaesthesiology 1997; 87: 63-7.
Figueredo E, Vivar-Diago M, Munoz-Blanco F. Laryngo-pharyngeal complaints after use of laryngeal mask airway. Can J Anesth 1999; 46: 220-5.
Marjot R. Pressure exerted by the laryngeal mask airway cuff upon the pharyngeal mucosa. Br J Anaesth 1993; 70: 25-9.
Inomata S, Nishikawa T, Suza A, Yamashita S. Transient bilateral vocal cord paralysis after insertion of a laryngeal mask airway. Anesthesiology 1995; 82: 787-8.
Nagai K, Sakuramoto C, Goto F. Unilateral hypoglossal nerve paralysis following the use of the laryngeal mask airway. Anaesthesia 1994; 49: 603-4.
King C, Street MK. Twelfth cranial nerve paralysis following use of the laryngeal mask airway. Anaesthesia 1994; 49: 786-7.
Rokamp KZ, Secher NH, Moller AM, Nielsen HB. Tracheal tube and laryngeal mask cuff pressure during anesthesia - mandatory monitoring is in need. BMC Anesthesiology 2010; 10: 20.
Lenoir RJ. Venous congestion of the neck; its relation to laryngeal mask cuff pressures. Br J Anaesth 2004; 93: 476-7.
Conflicts of interest
None declared.
Funding sources
This study is supported in part by the Department of Anesthesia, Toronto Western Hospital, University Health Network, University of Toronto, and by the Toronto General and Western Hospital Foundation, Toronto, Canada. Material support of the CPV airway was provided free of charge by AES, Inc., USA.
IRB Information
University Health Network, Research Ethics Board, 700 Bay Street, LuCliff Place, 17th Floor 1700-1, Toronto, ON, Canada M5G 1Z6 Phone: 416-581-7849.
Author information
Authors and Affiliations
Corresponding author
Additional information
Author contributions
David T. Wong and Frances F. Chung were involved in the study conception and design. David T. Wong, Vanita Mehta, Raviraj Raveendran, and Waleed Riad participated in data acquisition. David T. Wong, Amanda D. Tam, and Frances F. Chung participated in data analysis. Vanita Mehta was involved with patient recruitment. David T. Wong, Amanda D. Tam, Vanita Mehta, Raviraj Raveendran, Waleed Riad, and Frances F. Chung were involved in manuscript preparation.
Rights and permissions
About this article
Cite this article
Wong, D.T., Tam, A.D., Mehta, V. et al. New supraglottic airway with built-in pressure indicator decreases postoperative pharyngolaryngeal symptoms: a randomized controlled trial. Can J Anesth/J Can Anesth 60, 1197–1203 (2013). https://doi.org/10.1007/s12630-013-0044-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-013-0044-2