Abstract
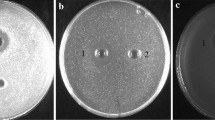
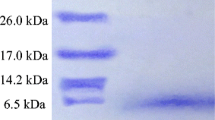
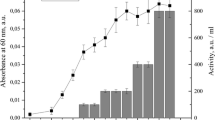
Enterocin LD3 was purified using activity-guided multistep chromatography techniques such as cation-exchange and gel-filtration chromatography. The preparation’s purity was tested using reverse-phase ultra-performance liquid chromatography. The specific activity was tested to be 187.5 AU µg−1 with 13-fold purification. Purified enterocin LD3 was heat stable up to 121 °C (at 15 psi pressure) and pH 2–6. The activity was lost in the presence of papain, reduced by proteinase K, pepsin and trypsin, but was unaffected by amylase and lipase, suggesting proteinaceous nature of the compound and no role of carbohydrate and lipid moieties in the activity. MALDI-TOF/MS analysis of purified enterocin LD3 resolved m/z 4114.6, and N-terminal amino acid sequence was found to be H2NQGGQANQ–COOH suggesting a new bacteriocin. Dissipation of membrane potential, loss of internal ATP and bactericidal effect were recorded when indicator strain Micrococcus luteus was treated with enterocin LD3. It inhibited Gram-positive and Gram-negative bacteria including human pathogens such as Staphylococcus aureus, Pseudomonas fluorescens, Pseudomonas aeruginosa, Salmonella typhi, Shigella flexneri, Listeria monocytogenes, Escherichia coli O157:H7, E. coli (urogenic, a clinical isolate) and Vibrio sp. These properties of purified enterocin LD3 suggest its applications as a food biopreservative and as an alternative to clinical antibiotics.





Similar content being viewed by others
References
Grande Burgos MJ, Pulido RP, Del Carmen López Aguayo M, Gálvez A, Lucas R (2014) The cyclic antibacterial peptide enterocin AS-48: isolation, mode of action, and possible food applications. Int J Mol Sci 15:22706–22727. doi:10.3390/ijms151222706
Perez RH, Zondo T, Sonomoto K (2014) Novel bacteriocins from lactic acid bacteria (LAB): various structures and applications. Microb Cell Fact 13:S3. doi:10.1186/1475-2859-13-S1-S3
Rumjuankiat K, Perez RH, Pilasombut K, Keawsompong S, Zendo T, Sonomoto K, Nitisinprasert S (2015) Purification and characterization of a novel plantaricin, KL-1Y, from Lactobacillus plantarum KL-1. World J Microbiol Biotechnol 31:983–994. doi:10.1007/s11274-015-1851-0
Sahoo TK, Jena PK, Patel AK, Seshadri S (2015) Purification and molecular characterization of the novel highly potent bacteriocin TSU4 produced by Lactobacillus animalis TSU4. Appl Biochem Biotechnol 177:90–104. doi:10.1007/s12010-015-1730-z
Arthur TD, Cavera VL, Chikindas ML (2014) On bacteriocin delivery systems and potential applications. Future Microbiol 9:235–248. doi:10.2217/fmb.13.148
De Arauz LJ, Jozala AF, Mazzola PG, Penna TCV (2009) Nisin biotechnological production and application: a review. Trends Food Sci Technol 20:146–154. doi:10.1016/j.tifs.2009.01.056
Vaillancourt K, LeBel G, Frenette M, Gottschalk M, Grenier D (2015) Suicin 3908, a new lantibiotic produced by a strain of Streptococcus suis serotype 2 isolated from a healthy carrier pig. PLoS ONE 10:e0117245. doi:10.1371/journal.pone.0117245
Agaliya PJ, Jeevaratnam K (2013) Characterization of the bacteriocins produced by two probiotic Lactobacillus isolates from idli batter. Ann Microbiol 4:1525–1535. doi:10.1007/s13213-013-0616-y
Bali V, Panesar PS, Bera MB (2014) Trends in utilization of agro-industrial byproducts for production of bacteriocins and their biopreservative applications. Crit Rev Biotechnol 28:1–11. doi:10.3109/07388551.2014.947916
Ennahar S, Sashihara T, Sonomoto K, Ishizaki A (2000) Class IIa bacteriocins: biosynthesis, structure and activity. FEMS Microbiol Rev 24:85–106. doi:10.1111/j.1574-6976.2000.tb00534.x
Hassan M, Kjos M, Nes IF, Diep DB, Lotfipour F (2012) Natural antimicrobial peptides from bacteria: characteristics and potential applications to fight against antibiotic resistance. J Appl Microbiol 113:723–736. doi:10.1111/j.1365-2672.2012.05338.x
Yount NY, Yeaman MR (2013) Peptide antimicrobials: cell wall as a bacterial target. Ann N Y Acad Sci 1277:127–138. doi:10.1111/nyas.12005
Brotz H, Beirbaum G, Markus A, Moliter E, Sahl HG (1995) Mode of action of the lantibiotic mersacidin: inhibition of peptidoglycan biosynthesis via a novel mechanism? Antimicrob Agents Chemother 39:714–719. doi:10.1128/AAC.39.3.714
Gupta A, Tiwari SK (2015) Probiotic potential of bacteriocin producing Enterococcus hirae strain LD3 isolated from batter of Dosa. Ann Microbiol 65:2333–2342. doi:10.1007/s13213-015-1075-4
deMan JC, Rogosa M, Sharpe M (1960) A medium for the cultivation of Lactobacilli. J Appl Bacteriol 23:130
Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principal of protein-dye binding. Anal Biochem 72:248–254. doi:10.1016/0003-2697(76)90527-3
Tiwari SK, Srivastava S (2008) Purification and characterization of a novel bacteriocin from natural isolate of Lactobacillus plantarum LR/14. Appl Microbiol Biotechnol 79:759–767. doi:10.1007/s00253-008-1482-6
Jimnez JJ, Borrero J, Diep DB, Gutiez L, Nes IF, Herranz C, Cintas LM, Hernandez PE (2013) Cloning, production, and functional expression of the bacteriocin sakacin A (SakA) and two SakA-derived chimeras in lactic acid bacteria (LAB) and the yeasts Pichia pastoris and Kluyveromyces lactis. J Ind Microbiol Biotechnol 40:977–993. doi:10.1007/s10295-013-1302-6
Kumar M, Tiwari SK, Srivastava S (2010) Purification and characterization of enterocin LR/6, a new bacteriocin from Enterococcus faecium LR/6. Appl Biochem Biotechnol 160:40–49. doi:10.1007/s12010-009-8586-z
Murdock C, Chikindas ML, Matthews KR (2010) The pepsin hydrolysate of bovine lactoferrin causes a collapse of the membrane potential in Escherichia coli O157:H7. Probiotics Antimicrob Proteins 2:112–119. doi:10.1007/s12602-010-9039-2
Tiwari SK, Noll SK, Cavera VL, Chikindas ML (2015) Improved antimicrobial activity of synthetic-hybrid bacteriocins designed from enterocin E50-52 and pediocin PA-1. Appl Environ Microbiol 81:1661–1667. doi:10.1128/AEM.03477-14
Gut IM, Blanke SR, van der Donk WA (2011) Mechanism of inhibition of Bacillus anthracis spore outgrowth by the lantibiotic nisin. ACS Chem Biol 6:744–752. doi:10.1021/cb1004178
Batdorj B, Dalgalarrondo M, Choiset Y, Pedroche J, Metro F, Prevost H, Chobert JM, Haertle T (2006) Purification and characterization of two bacteriocins produced by lactic acid bacteria isolated from Mongolian airag. J Appl Microbiol 101:837–848. doi:10.1111/j.1365-2672.2006.02966.x
Tiwari SK, Srivastava S (2008) Characterization of a bacteriocin from Lactobacillus plantarum strain LR/14. Food Biotechnol 22:241–267. doi:10.1080/08905430802262582
Gautam N, Sharma N, Ahlawat OP (2014) Purification and characterization of bacteriocin produced by Lactobacillus brevis UN isolated from Dhulliachar: a traditional food product of North East India. Indian J Microbiol 54:185–189. doi:10.1007/s12088-013-0427-7
Rushdy AA, Gomaa EZ (2013) Antimicrobial compounds produced by probiotic Lactobacillus brevis isolated from dairy products. Ann Microbiol 63:81–90. doi:10.1007/s13213-012-0447-2
Gaaloul N, Ben Braiek O, Hani K, Volski A, Chikindas ML, Ghrairi T (2015) Isolation and characterization of large spectrum and multiple bacteriocin-producing Enterococcus faecium strain from raw bovine milk. J Appl Microbiol 21:343–355. doi:10.1111/jam.12699
Gupta A, Tiwari SK (2014) Plantaricin LD1: a bacteriocin produced by food isolate of Lactobacillus plantarum LD1. Appl Biochem Biotechnol 172:3354–3362. doi:10.1007/s12010-014-0775-8
Ariel II, Budiman C, Jenie BS, Andreas E, Yuneni A (2015) Plantaricin IIA-1A5 from Lactobacillus plantarum IIA-1A5 displays bactericidal activity against Staphylococcus aureus. Benef Microbes 25:1–11. doi:10.3920/BM2014.0064
Barbour A, Philip K, Muniandy S (2013) Enhanced production, purification, characterization and mechanism of action of salivaricin 9 lantibiotic produced by Streptococcus salivarius NU10. PLoS ONE 8:e77751. doi:10.1371/journal.pone.0077751
Moll GN, Konings WN, Driessen AJ (1999) Bacteriocins: mechanism of membrane insertion and pore formation. Antonie Van Leeuwenhoek 76:185–198
Mathur H, Rea MC, Cotter PD, Ross RP, Hill C (2014) The potential for emerging therapeutic options for Clostridium difficile infection. Gut Microbes. doi:10.4161/19490976.2014.983768
Acknowledgments
The present work and AG were financially supported by University Grant Commission, New Delhi, under major research project (No. 36-38/2008 SR). SKT was supported by Indo-US Science and Technology Forum, New Delhi, and was truly acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Gupta, A., Tiwari, S.K., Netrebov, V. et al. Biochemical Properties and Mechanism of Action of Enterocin LD3 Purified from Enterococcus hirae LD3. Probiotics & Antimicro. Prot. 8, 161–169 (2016). https://doi.org/10.1007/s12602-016-9217-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12602-016-9217-y