Abstract
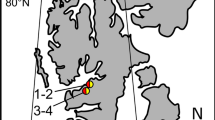
Red and green snow caused by snow algal blooms is common on glaciers and snowfields worldwide. Reddish and greenish snow samples containing algae were collected at the vicinity of penguin rockeries on King George Island (62°13′S, 58°47′W, near the King Sejong Station), Antarctica in February 2017 to investigate their physiology. Eight pigments and six fatty acids were detected from the samples. No difference in pigment and fatty acid (FA) composition was found between reddish and greenish snow samples. In contrast, spectral profiling and mycosporine-like amino acids (MAAs) were different between reddish and greenish snow. Particularly in greenish snow, a high absorbance between 450–600 nm was observed. The average MAA concentration was 316.0 μg g-1 in greenish snow, which was higher than that of reddish snow (278.2 μg g-1). The MAA to Particulate organic carbon (POC) ratio (mg (g C)-1) for reddish snow (6.2 mg (g C)-1) was higher than that of greenish snow (2.6 mg (g C)-1). These results suggest that reddish and greenish snow are considered to be the same species based on pigment and FA composition. Compared with photoprotective pigments, MAAs offer snow algae a more effective photoprotection strategy to promote tolerance of natural levels of ultraviolet radiation (UVR).
Similar content being viewed by others
References
Ahlgren G, Gustafsson I-B, Boberg M (1992) Fatty acid content and chemical composition of freshwater microalgae. J Phycol 28:37–50
Barlow RG,Mantoura RFC, Gough MA, Fileman TW (1993) Pigment signatures of the phytoplankton composition in the northeastern Atlantic during the 1990 spring bloom. Deep-Sea Res Pt II 40(1):459–477
Bidigare RR, Ondrusek ME, Kennicutt II,MC, Iturriaga R, Harvey HR, Hoham RW, Macko SA (1993) Evidence for a photoprotective function for secondary carotenoids of snow algae. J Phycol 29(4):427–434
Christian B, Lichti B, Pulz O, Grewe C, Luckas B (2009) Fast and unambiguous determination of EPA and DHA content in oil of selected strains of algae and cyanobacteria. Acta Agron Hung 57:249–253
Demmig-Adams B, Adams WW (1996) The role of xanthophyll cycle carotenoids in the protection of photosynthesis. Trends Plant Sci 1:21–26
Duval B, Shetty K, Thomas WH (2000) Phenolic compounds and antioxidant properties in the snow alga Chlamydomonas nivalis after exposure to UV light. J Appl Phycol 11:559–566
Goldman JC, McCarthy JJ, Peavey DG (1979) Growth rate influence on the chemical composition of phytoplankton in oceanic waters. Nature 279:210–215
Goss R, Jakob T (2010) Regulation and function of xanthophyll cycle-dependent photoprotection in algae. Photosynth Res 106:103–122
Ha S-Y, La HS, Min J-O, Chung K-H, Kang S-H, Shin K-H (2014a) Photoprotective function of mycosporine-like amino acids in a bipolar diatom (Porosira glacialis): evidence from ultraviolet radiation and stable isotope probing. Diatom Res 29(4):399–409. doi:10.1080/0269249X.2014.894945
Ha S-Y, Min J-O, Joo HM, Chung KH (2014b) Production rate estimation of mycosporine-like amino acids in two Arctic melt ponds by stable isotope probing with NaH13CO3. J Phycol 50:901–907
Häder D-P, Kumar HD, Smith RC, Worrest RC (1998) Effects on aquatic ecosystems. J Photoch Photobio B 46:53–68
Hama T, Handa N (1987) Pattern of organic matter production in natural phytoplankton population in a eutrophic lake 1. Intracellular products. Arch Hydrobiol 109:107–120
Hamilton TL, Havig J (2017) Primary productivity of snow algae communities on stratovolcanoes of the Pacific Northwest. Geobiology 15:280–295
Hodson AJ, Nowak A, Cook J, Sabacka M, Wharfe ES, Pearce DA, Convey P, Vieira G (2017) Microbes influence the biogeochemical and optical properties of maritime Antarctic snow. J Geophys Res-Biogeos 122(6):1456–1470. doi:10.1002/2016JG003694
Holzinger A, Allen MC, Deheyn DD (2016) Hyperspectral imaging of snow algae and green algae from aeroterrestrial habitats. J Photoch Photobio B 162:412–420
Jeffrey SW, Mantoura RFC, Bjornland T (1997) Data for the identification of 47 key phytoplankton pigments. In: Jeffrey SW, Mantoura RFC, Wright SW (eds) Phytoplankton pigments in oceanography: guidelines to modern methods. Monographs on Oceanographic Methodology, UNESCO, Paris, pp 449–559
Karentz D (2001) Chemical defenses of marine organisms against solar radiation exposure: UV-absorbing mycosporine-like amino acids and scytonemin. In: McClintock JB, Baker BJ (eds) Marine chemical ecology. CRC Press, Boca Raton, pp 481–519
Layman CA, Araujo MS, Boucek R, Hammerschlag-Peyer CM, Harrison E, Jud ZR. Matich P, Rosenblatt AE, Vaudo JJ, Yeager LA, Post DM, Bearhop S (2012) Applying stable isotopes to examine food-webs tructure: an overview of analytical tools. Biol Rev 87:545–562
Lemoine Y, Schoefs B (2010) Secondary ketocarotenoid astaxanthin biosynthesis in algae: a multifunctional response to stress. Photosynth Res 106:155–177
Leya T, Rahn A, Lutz C, Remias D (2009) Response of arctic snow and permafrost algae to high light and nitrogen stress by changes in pigment composition and applied aspects for biotechnology. FEMS Microbiol Ecol 67:432–443
Ling HU, Seppelt RD (1990) Snow algae of the Windmill Islands, continental Antarctica. Mesotaenium berggrenii (Zygnematales, Chlorophyta) the alga of grey snow. Antarct Sci 2(2):143–148
Llewellyn CA, Harbour DS (2003) A temporal study of mycosporinelike amino acids in surface water phytoplankton from the English channel and correlation with solar irradiation. J Mar Biol Assoc UK 83:1–9
Lutz S, Anesio AM, Field K, Benning LG (2015) Integrated ‘Omics’, targeted metabolite and single-cell analyses of arctic snow algae functionality and adaptability. Front Microbiol 6:1323
Lutz S, Anesio AM, Jorge Villar SE (2014) Variations of algal communities cause darkening of a Greenland glacier. FEMS Microbiol Ecol 89(2):402–414
Lutz S, Anesio AM, Raiswell R, Edwards A, Newton RJ, Gill F, Benning LG (2016) The biogeography of red snow microbiomes and their role in melting arctic glaciers. Nature Commun 7:11698. doi:10.1038/ncomms11968
Moline MA, Claustre H, Frazer TK, Schofield O, Vernet M (2004) Alteration of the food web along the Antarctic Peninsula in response to a regional warming trend. Global Change Biol 10:1973–1980. doi:10.1111/j.1365-2486.2004.00825
Moon H-W, Rauhan WM, Hussin W, Kim H-C, Ahn I-Y (2015) The impacts of climate change on Antarctic nearshore megaepifaunal benthic assemblages in a glacial fjord on King George Island: responses and implications. Ecol Indi 57:280–292
Müller T, Bleib W, Martin C-D, Rogaschewski S, Fuhr G (1998) Snow algae from northwest Svalbard: their identification, distribution, pigment and nutrient content. Polar Biol 20(1):14–32
Neale PJ, Banaszak AT, Jarriel CR (1998) Ultraviolet sunscreens in Gymnodinium sanguineum (Dinophyceae): mycosporinelike amino acids protect against inhibition of photosynthesis. J Phycol 34:928–938
Park MO (2006) Composition and distribution of phytoplankton with size fraction results at Southwestern East/Japan Sea. Ocean Sci J 41:301–313
Pereira SL, Leonard AE, Huang YS, Chuang LT, Mukerji P (2004) Identification of two novel microalgal enzymes involved in the conversion of the ω3-fatty acid, eicosapentaenoic acid, into docosahexaenoic acid. Biochem J 384:357–366
Peterson BJ, Fry B (1987) Stable isotopes in ecosystem studies. Annu Rev Ecol Syst 18:293–320
Pritchard HD, Ligtenberg SRM, Frickers HA, Vaughan DG, van den Broeke MR, Padman L (2012) Antarctic ice-sheet loss driven by basal melting of ice shelves. Nature 484:502–505. doi:10.1038/nature10968
Redfield AC (1958) The biological control of chemical factors in the environment. Am Sci 46:205–221
Remias D, Lütz-Meindl U, Lütz C (2005) Photosynthesis, pigments and ultrastructure of the alpine snowalga Chlamydomonas nivalis. Eur J Phycol 40(3):259–268
Remias D, Pichrtová M, Pangratz M, Lütz C, Holzinger A (2016) Ecophysiology, secondary pigments and ultrastructure of Chlainomonas sp. (Chlorophyta) from the European Alps compared with Chlamydomonas nivalis forming red snow. FEMS Micorbiol Ecol 92(4):fiw030. doi:10.1093/femsec/fiw030
Remias D, Wastian H, Lütz C, Leya T (2013) Insight into the biology and phylogeny of Chloromonas polyptera (Chlorophyta), an alga causing orange snow in Maritime Antarctica. Antarct Sci 25(5):648–656
Řezanka T, Nedbalová L, Sigler K (2008) Unusual medium-chain polyunsaturated fatty acids from the snow alga Chloromonas brevispina. Microbiol Res 163:373–379
Rott H, Rack W, Skvarca P, de Angelis H (2002) Northern larsen ice shelf, Antarctica: further retreat after collapse. Ann Glaciol 34:277–282
Rückamp M, Braun M, Suckro S, Blindow N (2011) Observed glacial changes on the King George Island ice cap, Antarctica, in the last decade. Global Planet Change 79:99–109
Sahade R, Lagger C, Torre L, Momo F, Monien P, Schloss I, Barnes DKA, Servetto N, Taratelli S, Tatián M, Zamboni N, Abele D (2015) Climate change and glacier retreat drive shifts invan Antarctic benthic ecosystem. Sci Adv 1(10):e1500050. doi:10.1126/sciadv. 1500050
Sahu A, Pancha I, Jain D, Paliwal C, Ghosh T, Patidar S, Bhattacharya S, Mishra S (2013) Fatty acids as biomarkers of microalgae. Phytochemistry 89:53–58
Sinha RP, Ambasht NK, Sinha JP, Klisch M, Häder DP (2003) UV-B-induced synthesis of mycosporine-like amino acids in three strains of Nodularia (cyanobacteria). J Photoch Photobio B 71:51–58
Spijkerman E, Wacker A, Weithoff G, Leya T (2012) Elemental and fatty acid composition of snow algae in Arctic habitats. Front Microbiol 3:380
Steinhart GS, Likens GE, Soto D (2002) Physiological indicators of nutrient deficiency in phytoplankton in southern Chilean lakes. Hydrobiologia 489:21–27
Takeuchi N (2013) Seasonal and altitudinal variations in snow algal communities on an Alaskan glacier (Gulkana glacier in the Alaska range). Environ Res Lett 8:035002. doi:10.1088/1748-9326/8/3/035002
Takeuchi N, Dial R, Kohshima S, Segawa T, Uetake J (2006) Spatial distribution and abundance of red snow algae on the Harding Icefield, Alaska derived from a satellite image. Geophys Res Lett 33:L21502. doi:10.1029/2006GL027819
Thomas WH, Duval B (1995) Sierra Nevada, California, U.S.A., snow algae: snow albedo changes, algal-bacterial interrelationships, and ultraviolet radiation effects. Arct Antarct Alp Res 27(4):389–399
Turner J, Barrand NE, Bracegirdle TJ, Convey P, Hodgson DA, Jarvis M, Jenkins A, Marshall GJ, Meredith MP, Roscoe HK, Shanklin JD, French J, Goosse H, Guglielmin M, Gutt J, Jacobs SS,Kennicutt MCI, Masson-Delmotte V, Mayewski P, Navarro F, Robinson S, Scambos T, Sparrow M, Speer K, Summerhayes CP, Klepikov AV (2014) Antarctic climate change and the environment: an update. Polar Rec 50(3):237–259
Volkmann M, Gorbushina AA (2006) A broadlyapplicable method for extraction and characterization of mycosporines and mycosporine-like amino acids ofterrestrial, marine and freshwater origin. FEMS Microbiol Lett 255:286–295
Wada E, Terazaki M, Kabaya Y, Nemoto T (1987) 15N and 13C abundances in the Antarctic Ocean with emphasis on the biogeochemical structure of the food web. Deep-Sea Res 34:829–841
Wada N, Sakamoto T, Matsugo S (2015) Mycosporine-like amino acids and their derivatives as natural antioxidants. Antioxidants 4(3):603–646
Whitehead K, Karentz D, Hedges JI (2001) Mycosporine-like amino acids (MAAs) in phytoplankton, a herbivorous pteropod (Limacina helicina), and its pteropod predator (Clione antarctica) in McMurdo Bay, Antarctica. Mar Biol 139:1013–1019
Whitelam GC, Codd GA (1986) Damaging effects of light on microorganisms. In: Herbert RA, Codd GA (eds) Microbes in extreme environments. Academic Press, London, pp 129–169
Yacobi YZ, Ostrovsky I (2012) Sedimentation of phytoplankton: role of ambient conditions and life strategies of algae. Hydrobiologia 698:111–120
Zapata M, Rodríguez F, Garrido JL (2000) Separation of chlorophylls and carotenoids from marine phytoplankton: a new HPLC method using a reversed phase C8 column and pyridinecontaining mobile phases. Mar Ecol-Prog Ser 195:29–45
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, B.K., Joo, H., Lee, B. et al. Physiological Characteristics and Related Biochemical Parameters of Snow Algae from King George Island, Antarctica. Ocean Sci. J. 53, 621–630 (2018). https://doi.org/10.1007/s12601-018-0053-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12601-018-0053-8