Abstract
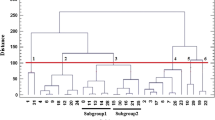
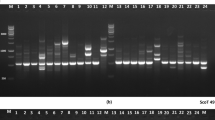
In order to provide information for further research and utilization of Erianthus arundinaceus (Retz.) Jesw., the phenotypic variation and genetic diversity in the collection of E. arundinaceus (Retz.) Jesw. germplasm in Guangxi, China were investigated. The coefficient of variation (CV) in four quantitative traits, plant height, stalk diameter, spikelet length and number of panicle nodes, of 50 E. arundinaceus (Retz.) Jesw. clones ranged from 12.71 to 16.14 %, stalk diameter had the highest coefficient of variation, followed by spikelet length, and number of panicle nodes showed the lowest. Fifteen start condon targeted polymorphism (SCoT) marker primers were used to assess the genetic diversity of the 50 collections of E. arundinaceus (Retz.) Jesw. germplasm. A total of 336 genotypes were investigated for SCoT polymorphism, which produced 284 amplicons with 83.97 % polymorphism, indicating that E. arundinaceus (Retz.) Jesw. germplasm had abundant diversity in Guangxi. The Jaccard’s similarity coefficients among these accessions varied from 0.61 to 0.88 with an average of 0.71. The UPGMA clustering analysis showed the 50 E. arundinaceus (Retz.) Jesw. accessions could be divided into four major groups at the similarity coefficient level 0.73. The UPGMA dendrogram reflected the geographic distribution patterns of these accessions.


Similar content being viewed by others
References
Cai, Q., K.S. Aitken, Y.H. Fan, G. Piperidis, X.L. Liu, C.L. McIntyre, X.Q. Huang, and P. Jackson. 2012. Assessment of the genetic diversity in a collection of Erianthus arundinaceus. Genetic Resources and Crop Evolution 59: 1483–1491.
Chen, H., X.H. He, G.X. Huang, F. Li, and J.C. Jiang. 2012. Comparison and analysis of SCoT and ISSR markers for genetic diversity of Longan. Guihaia 32(4): 536–541.
Chen, X.L., Y.R. Li, L.T. Yang, J.M. Wu, C. Luo, F.Q. Xiong, and L. Yang. 2010. cDNA-SCoT differential display of cold resistance related genes in sugarcane under low temperature stress. Biotechnology Bulletin 8: 120–124.
Collard, B.C.Y., and D.J. Mackill. 2009. Start codon targeted (SCoT) polymorphism: A simple, novel DNA marker technique for generating gene targeted markers in plants. Plant Molecular Biology Reporter 27: 86–93.
Feng, S.G., R.F. He, M.Y. Jiang, J.J. Lu, X.X. Shen, J.J. Liu, Z.A. Wang, and H.Z. Wang. 2016. Genetic diversity and relationships of medicinal Chrysanthemum morifolium revealed by start codon targeted (SCoT) markers. Scientia Horticuturae 201: 118–123.
Grivet, L., and P. Arruda. 2001. Sugarcane genomics: depicting the complex genome of an important tropical crop. Current Opinion in Plant Biology 5: 122–127.
Hu, R., M.Z. Bao, X.Q. Wu, H.S. Tan, and X.P. Fu. 2015. Analyses and utilization of the phenotypic diversity of carnation. Journal of Huangzhong Agricultural University 34(2): 16–23.
Kalwade, S.B., and R.M. Devarumath. 2014. Single strand conformation polymorphism of genomic and EST-SSRs marker and its utility in genetic evaluation of sugarcane. Physiol Mol Biol Plants 20(3): 313–321.
Li, Y.R. 2010. Modern Sugarcane Science, 61–125. Beijing: China Agriculture Press.
Liu, X.H., F.X. Fang, R.H. Zhang, H.Z. Song, R.Z. Yang, Y.J. Gao, H.P. Ou, J.C. Lei, T. Luo, W.X. Duan, G.M. Zhang, and Y.R. Li. 2012a. Identification of progenies from sugarcane × Narenga porphyrocoma (Hance) Bor. by SSR marker. Southwest China. Journal of Agricultural Science 25(1): 38–42.
Liu, X.H., F.X. Feng, R.H. Zhang, H.Z. Song, R.Z. Yang, Y.J. Gao, J.C. Lei, T. Luo, W.X. Duan, and G.M. Zhang. 2012b. Identification and genetic analysis of hybrid from cross between Erianthus arundinaceum (Retz.) Jesw. and Saccharum spontaneum L. Acta Agronomica Sinica 32(6): 11–15.
Liu, Y., X.L. Liu, H.B. Liu, Y.L. Yao, and J.B. Su. 2013. Collection and genetic diversity of wild germplasm resource in sugarcane from Hainan Province. Chinese Agricultural Science Bulletin 29(1): 199–208.
Luo, T., H.X. Yang, H.F. Qin, X.H. Xu, Y.J. Gao, W.X. Duan, R.H. Zhang, X.H. Liu, H.Z. Song, Y.X. Huang, and G.M. Zhang. 2013. Application of SCoT molecular marker in construction of molecular genetic linkage mao of Saccharum spontaneun L. Journal of Plant Genetic Resources 14(4): 704–710.
Nair, N.V., A.W. Jebadhas, and T.V. Sreenivasan. 1993. Saccharum germplasm collections in Arunachal Pradesh. Indian Journal of Plant Genetic Resources 6(1): 21–26.
Nerkar, G., F. Farsangi, and R. Devarumath. 2015. Organellar genome diversity in Saccharum and Erianthus spp. Revealed by PCR-RFLP. Molecular Plant Breeding 6(11): 1–11.
Priya, M.S., K.H.P. Reddy, M.H. Kumat, V. Rajarjeswari, G.M. Naidu, R. Narasimhulu, B. Reddy, and R. Kumar. 2005. Genetic diversity and character aassociation among sugarcane (Saccharum Spp.) clones. Bioinfolet 12: 444–451.
Que, Y.X., Y.B. Pan, Y.H. Lu, C. Yang, Y.T. Yang, N. Huang, and L.P. Xu. 2014. Genetic analysis of diversity within a Chinese local sugarcane germplasm based on start codon targeted polymorphism. BioMed Research International 350: 1–10.
Rajesh, M.K., A.A. Sabna, K.E. Rachana, S. Rahman, B.A. Jerard, and A. Karun. 2015. Genetic relationship and diversity among coconut (Cocos nucifera L.) accessions revealed through SCoT analysis. Biotechnology 5: 999–1006.
Rao, V.P., R. Chaudhary, S. Singh, R.S. Sengar, and V. Sharma. 2014. Assessment of genetic diversity analysis in contrasting sugarcane varieties using random amplified polymorphic DNA (RAPD) markers. African Journal of Biotechnology 13(37): 3736–3741.
Satya, P., M. Karan, S. Jana, S. Mitra, A. Sharma, P.G. Karmakar, and D.P. Ray. 2015. Start codon targeted (SCoT) polymorphism reveal genetic diversity in wild and domesticated populations of ramie (Boehmeria nivea L. Gaudich.), a premium textile fible producting species. Meta Gene 3: 62–70.
Sharma, M.D., U. Dobhal, P. Singh, S. Kumar, A.K. Gaur, S.P. Singh, A.S. Jeena, E.P. Koshy, and S. Kumar. 2014. Assessment of genetic diversity among sugarcane cultivars using novel microsatellite marker. African Journal of Biotechnology 13(13): 1444–1451.
Song, S., Y.W. Wu, X.B. Ji, L.Q. Song, and X.P. Wen. 2013. Genetic analysis of apple rootstock germplasm from Guizhou province using ISSR markers. Journal of Huazhong Agricultural University 23(1): 19–24.
Su, Y.C., H. Lin, H.B. Wang, Y.X. Que, Q.B. Wu, S.S. Chen, and L.P. Xu. 2012. Optimization of SCoT-PCR reaction system, and screening and utilization of polymorphic primers in sugarcane. Chinese Journal of Applied and Environmental Biology 18(5): 810–818.
Wang, J.S., Z.X. Liu, F. Fan, L.P. Han, and G.H. Xie. 2012. Analysis of genetic diversity and inheritability of agronomic traits and chemical compositions in sweet sorghun (sorghum bicolour). Journal of China Agricultural University 17(6): 83–91.
Wei, Y.L., X.H. He, C. Luo, and H. Chen. 2012. Genetic diversity of podocarpus by SCoT markers. Guihaia 32(1): 90–93.
Wu, J.M., Y.R. Li, A.Q. Wang, L. Yang, and L.T. Yang. 2010. Different expression of gibberellin-induced genes for stalk elongation of sugarcane analysis with cDNA–ScoT. Acta Agronomica Sinica 36(11): 1883–1890.
Wu, K.C., Y.R. Li, L.T. Yang, and J.M. Wu. 2013. Establishment, optimization and application of cDNA-SCoT reaction system in sugarcane. Chinese Journal of Tropical Crops 34(5): 892–898.
Xiong, F.Q., J. Jiang, R.C. Zhong, Z.H. Qiang, L.Q. He, Z. Li, W.J. Zhuang, and R.H. Tang. 2010. Application SCoT molecular marker in Genus arachis. Acta Agronomica Sinica 36(12): 2055–2061.
Xiong, F.Q., R.C. Zhong, Z.Q. Han, J. Jiang, L.Q. He, W.J. Zhuang, and R.H. Tang. 2011. Start codon targeted polymorphism for evaluation of functional genetic variation and relationships in cultivated peanut (Arachis hypogaea L.) genotypes. Molecular Biology Reports 38: 3487–3494.
You, Q., Y.B. Pan, L.P. Xu, S.W. Gao, Q.N. Wang, Y.C. Su, Y.Q. Yang, Q.B. Wu, D.G. Zhou, and Y.X. Que. 2015. Genetic diversity analysis of sugarcane germplasm based on fluorescence-labeled simple sequence repeat markers and a capillary electrophoresis-based genotyping platform. Sugar Tech. doi:10.1007/s12355-015-0395-9.
Zan, F.G., X.M. Ying, C.W. Wu, P.F. Zhao, X.K. Chen, L. Ma, H.S. Su, and J.Y. Liu. 2015. Genetic diversity of 98 collections of sugarcane germplasm with AFLP markers. Scientia Agricultura Sinica 48(5): 1002–1010.
Zhang, J.B., J.J. Yan, S.Q. Bai, Y.W. Zhang, Z.X. Dao, D.X. Li, C.B. Zhang, M.H. You, Y. Zhang, J. Zhang, and F.Y. Yang. 2013. Genetic diversity of native Erianthus arundinaceus germplasm detected by SRAP markers. Jourmal of Agricultural Biotechnology 21(10): 1193–1202.
Zhang, Y., J.J. Yan, S.Q. Bai, Y. Peng, X.Z. Liang, J.B. Zhang, J. Zhang, and C. Hu. 2015. SRAP analysis of genetic structure of wide Erianthus arundinaceum populations in Sichuan China. Chinese Journal of Grassland 37(1): 15–21.
Zhao, R.Q., Y.H. Gao, X.L. Zhang, and J.P. Si. 2012. Establishment and optimization of SCoT-PCR reaction system for Dendrobium officinale. Acta Agriculturae Nucleatae Sinica 26(4): 648–655.
Funding
This research was financially supported by the National High Technology Research and Development Program of China (863 Program) (2013AA102604), National Natural Science Foundation of China (31101195), Guangxi Special Funds for Bagui Scholars and Distinguished Experts (2013), Funds of Guangxi (2014GXNSFBA118118, 2012GXNSFBA053048, 14123001-1-4, 15-140-13), Fund for the Guangxi Innovation Teams of Modern Agriculture Technology (gjnytxgxcxtd-03) and Fund of Guangxi Academy of Agricultural Sciences (2016JM07).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Xi-Hui Liu and Huan-Zhong Song have contributed equally in preparation of this manuscript.
Rights and permissions
About this article
Cite this article
Liu, XH., Song, HZ., Zhang, GM. et al. Phenotypic Variation and Genetic Diversity in the Collections of Erianthus Arundinaceus (Retz.) Jesw.. Sugar Tech 19, 359–367 (2017). https://doi.org/10.1007/s12355-016-0475-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12355-016-0475-5