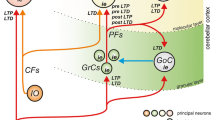
Abstract
The mechanism by which a learnt synaptic weight change can contribute to learning or adaptation of brain function is a type of credit assignment problem, which is a key issue for many parts of the brain. In the cerebellum, detailed knowledge not only of the local circuitry connectivity but also of the topography of different sources of afferent/external information makes this problem particularly tractable. In addition, multiple forms of synaptic plasticity and their general rules of induction have been identified. In this review, we will discuss the possible roles of synaptic and cellular plasticity at specific locations in contributing to behavioral changes. Focus will be on the parts of the cerebellum that are devoted to limb control, which constitute a large proportion of the cortex and where the knowledge of the external connectivity is particularly well known. From this perspective, a number of sites of synaptic plasticity appear to primarily have the function of balancing the overall level of activity in the cerebellar circuitry, whereas the locations at which synaptic plasticity leads to functional changes in terms of limb control are more limited. Specifically, the postsynaptic forms of long-term potentiation (LTP) and long-term depression (LTD) at the parallel fiber synapses made on interneurons and Purkinje cells, respectively, are the types of plasticity that mediate the widest associative capacity and the tightest link between the synaptic change and the external functions that are to be controlled.


Similar content being viewed by others
References
van Kan PL, Gibson AR, Houk JC. Movement-related inputs to intermediate cerebellum of the monkey. J Neurophysiol. 1993;69(1):74–94.
van Kan PL, Houk JC, Gibson AR. Output organization of intermediate cerebellum of the monkey. J Neurophysiol. 1993;69(1):57–73.
Armstrong DM, Edgley SA. Discharges of Purkinje cells in the paravermal part of the cerebellar anterior lobe during locomotion in the cat. J Physiol. 1984;352:403–24.
Pasalar S et al. Force field effects on cerebellar Purkinje cell discharge with implications for internal models. Nat Neurosci. 2006;9(11):1404–11.
Spanne A et al. Spike generation estimated from stationary spike trains in a variety of neurons in vivo. Front Cell Neurosci. 2014;8:199.
Ito M, Sakurai M, Tongroach P. Climbing fibre induced depression of both mossy fibre responsiveness and glutamate sensitivity of cerebellar Purkinje cells. J Physiol. 1982;324:113–34.
Ekerot CF, Kano M. Long-term depression of parallel fibre synapses following stimulation of climbing fibres. Brain Res. 1985;342(2):357–60.
Jorntell H, Ekerot CF. Reciprocal bidirectional plasticity of parallel fiber receptive fields in cerebellar Purkinje cells and their afferent interneurons. Neuron. 2002;34(5):797–806.
Coesmans M et al. Bidirectional parallel fiber plasticity in the cerebellum under climbing fiber control. Neuron. 2004;44(4):691–700.
Lev-Ram V et al. A new form of cerebellar long-term potentiation is postsynaptic and depends on nitric oxide but not cAMP. Proc Natl Acad Sci U S A. 2002;99(12):8389–93.
Jorntell H, Ekerot CF. Receptive field plasticity profoundly alters the cutaneous parallel fiber synaptic input to cerebellar interneurons in vivo. J Neurosci. 2003;23(29):9620–31.
Rancillac A, Crepel F. Synapses between parallel fibres and stellate cells express long-term changes in synaptic efficacy in rat cerebellum. J Physiol. 2004;554(Pt 3):707–20.
Tanaka S et al. Long-term potentiation of inhibitory synaptic transmission onto cerebellar Purkinje neurons contributes to adaptation of vestibulo-ocular reflex. J Neurosci. 2013;33(43):17209–20.
Kano M, Fukunaga K, Konnerth A. Ca(2+)-induced rebound potentiation of gamma-aminobutyric acid-mediated currents requires activation of Ca2+/calmodulin-dependent kinase II. Proc Natl Acad Sci U S A. 1996;93(23):13351–6.
Ichise T et al. mGluR1 in cerebellar Purkinje cells essential for long-term depression, synapse elimination, and motor coordination. Science. 2000;288(5472):1832–5.
Weber JT et al. Long-term depression of climbing fiber-evoked calcium transients in Purkinje cell dendrites. Proc Natl Acad Sci U S A. 2003;100(5):2878–83.
Belmeguenai A et al. Intrinsic plasticity complements long-term potentiation in parallel fiber input gain control in cerebellar Purkinje cells. J Neurosci. 2010;30(41):13630–43.
Xu W, Edgley SA. Climbing fibre-dependent changes in Golgi cell responses to peripheral stimulation. J Physiol. 2008;586(Pt 20):4951–9.
Mapelli J, D′Angelo E. The spatial organization of long-term synaptic plasticity at the input stage of cerebellum. J Neurosci. 2007;27(6):1285–96.
Leiras R et al. Processing afferent proprioceptive information at the main cuneate nucleus of anesthetized cats. J Neurosci. 2010;30(46):15383–99.
Ekerot CF, Larson B. Correlation between sagittal projection zones of climbing and mossy fibre paths in cat cerebellar anterior lobe. Brain Res. 1973;64:446–50.
Cooke JD et al. Organization of afferent connections to cuneocerebellar tract. Exp Brain Res. 1971;13(4):359–77.
Cooke JD et al. Origin and termination of cuneocerebellar tract. Exp Brain Res. 1971;13(4):339–58.
Edin B. Cutaneous afferents provide information about knee joint movements in humans. J Physiol. 2001;531(Pt 1):289–97.
Edin BB. Quantitative analysis of static strain sensitivity in human mechanoreceptors from hairy skin. J Neurophysiol. 1992;67(5):1105–13.
Jorntell H, Ekerot CF. Properties of somatosensory synaptic integration in cerebellar granule cells in vivo. J Neurosci. 2006;26(45):11786–97.
Garwicz M, Jorntell H, Ekerot CF. Cutaneous receptive fields and topography of mossy fibres and climbing fibres projecting to cat cerebellar C3 zone. J Physiol. 1998;512(Pt 1):277–93.
Spanne A, Jorntell H. Processing of multi-dimensional sensorimotor information in the spinal and cerebellar neuronal circuitry: a new hypothesis. PLoS Comput Biol. 2013;9(3):e1002979.
Oscarsson O. Functional organization of spinocerebellar paths, in Handbook of Sensory Physiology. In: Iggo A, editor. New York: Springer-Verlag; 1973. p. 339–380.
Illert M, Lundberg A, Tanaka R. Integration in descending motor pathways controlling the forelimb in the cat. 2. Convergence on neurones mediating disynaptic cortico-motoneuronal excitation. Exp Brain Res. 1976;26(5):521–40.
Jankowska E, Nilsson E, Hammar I. Processing information related to centrally initiated locomotor and voluntary movements by feline spinocerebellar neurones. J Physiol. 2011;589(Pt 23):5709–25.
Illert M et al. Integration in descending motor pathways controlling the forelimb in the cat. 7. Effects from the reticular formation on C3-C4 propriospinal neurones. Exp Brain Res. 1981;42(3–4):269–81.
Alstermark B, Isa T. Circuits for skilled reaching and grasping. Annu Rev Neurosci. 2012;35:559–78.
Santello M, Baud-Bovy G, Jorntell H. Neural bases of hand synergies. Front Comput Neurosci. 2013;7:23.
Brodal P, Walberg F. The pontine projection to the crebellar anterior lobe. An experimental study in the cat with retrograde transport of horseradish peroxidase. Exp Brain Res. 1977;29(2):233–48.
Lisberger SG, Fuchs AF. Role of primate flocculus during rapid behavioral modification of vestibuloocular reflex. II. Mossy fiber firing patterns during horizontal head rotation and eye movement. J Neurophysiol. 1978;41(3):764–77.
Noda H, Suzuki DA. Processing of eye movement signals in the flocculus of the monkey. J Physiol. 1979;294:349–64.
Escudero M, Cheron G, Godaux E. Discharge properties of brain stem neurons projecting to the flocculus in the alert cat. II. Prepositus hypoglossal nucleus. J Neurophysiol. 1996;76(3):1775–85.
Cheron G et al. Existence in the nucleus incertus of the cat of horizontal-eye-movement-related neurons projecting to the cerebellar flocculus. J Neurophysiol. 1995;74(3):1367–72.
Prsa M et al. Characteristics of responses of Golgi cells and mossy fibers to eye saccades and saccadic adaptation recorded from the posterior vermis of the cerebellum. J Neurosci. 2009;29(1):250–62.
Jorntell H et al. Segregation of tactile input features in neurons of the cuneate nucleus. Neuron. 2014;83(6):1444–52.
Bengtsson F, Jorntell H. Sensory transmission in cerebellar granule cells relies on similarly coded mossy fiber inputs. Proc Natl Acad Sci U S A. 2009;106(7):2389–94.
Huang CC et al. Convergence of pontine and proprioceptive streams onto multimodal cerebellar granule cells. Elife. 2013;2:e00400.
Marr D. A theory of cerebellar cortex. J Physiol. 1969;202(2):437–70.
Albus JS. A theory of cerebellar function. Math Biosci. 1971;10:25–61.
Geborek P et al. Cerebellar cortical neuron responses evoked from the spinal border cell tract. Front Neural Circ. 2013;7:157.
Ekerot CF, Jorntell H. Parallel fibre receptive fields of Purkinje cells and interneurons are climbing fibre-specific. Eur J Neurosci. 2001;13(7):1303–10.
Geborek P, Bengtsson F, Jorntell H. Properties of bilateral spinocerebellar activation of cerebellar cortical neurons. Front Neural Circ. 2014;8:128–37.
Roggeri L et al. Tactile stimulation evokes long-term synaptic plasticity in the granular layer of cerebellum. J Neurosci. 2008;28(25):6354–9.
Gebre SA, Reeber SL, Sillitoe RV. Parasagittal compartmentation of cerebellar mossy fibers as revealed by the patterned expression of vesicular glutamate transporters VGLUT1 and VGLUT2. Brain Struct Funct. 2012;217(2):165–80.
Tolbert DL, Alisky JM, Clark BR. Lower thoracic upper lumbar spinocerebellar projections in rats: a complex topography revealed in computer reconstructions of the unfolded anterior lobe. Neuroscience. 1993;55(3):755–74.
Holtzman T et al. Cerebellar Golgi cells in the rat receive multimodal convergent peripheral inputs via the lateral funiculus of the spinal cord. J Physiol. 2006;577(Pt 1):69–80.
Holtzman T et al. Multiple extra-synaptic spillover mechanisms regulate prolonged activity in cerebellar Golgi cell-granule cell loops. J Physiol. 2011;589(Pt 15):3837–54.
Brickley SG, Cull-Candy SG, Farrant M. Development of a tonic form of synaptic inhibition in rat cerebellar granule cells resulting from persistent activation of GABAA receptors. J Physiol. 1996;497(Pt 3):753–9.
Bengtsson F, Geborek P, Jorntell H. Cross-correlations between pairs of neurons in cerebellar cortex in vivo. Neural Netw. 2013;47:88–94.
Duguid I et al. Tonic inhibition enhances fidelity of sensory information transmission in the cerebellar cortex. J Neurosci. 2012;32(32):11132–43.
Bengtsson F, Svensson P, Hesslow G. Feedback control of Purkinje cell activity by the cerebello-olivary pathway. Eur J Neurosci. 2004;20(11):2999–3005.
Chaumont J et al. Clusters of cerebellar Purkinje cells control their afferent climbing fiber discharge. Proc Natl Acad Sci U S A. 2013;110(40):16223–8.
Bengtsson F, Hesslow G. Cerebellar control of the inferior olive. Cerebellum. 2006;5(1):7–14.
Cheron G et al. BK channels control cerebellar Purkinje and Golgi cell rhythmicity in vivo. PLoS One. 2009;4(11):e7991.
Sausbier M et al. Cerebellar ataxia and Purkinje cell dysfunction caused by Ca2 + −activated K+ channel deficiency. Proc Natl Acad Sci U S A. 2004;101(25):9474–8.
Fremont R et al. Abnormal high-frequency burst firing of cerebellar neurons in rapid-onset dystonia-parkinsonism. J Neurosci. 2014;34(35):11723–32.
Chen G et al. Low-frequency oscillations in the cerebellar cortex of the tottering mouse. J Neurophysiol. 2009;101(1):234–45.
Hashimoto K, Kano M. Functional differentiation of multiple climbing fiber inputs during synapse elimination in the developing cerebellum. Neuron. 2003;38(5):785–96.
Hansel C, Linden DJ. Long-term depression of the cerebellar climbing fiber–Purkinje neuron synapse. Neuron. 2000;26(2):473–82.
Jorntell H, Hansel C. Synaptic memories upside down: bidirectional plasticity at cerebellar parallel fiber-Purkinje cell synapses. Neuron. 2006;52(2):227–38.
Mandolesi G et al. GluRdelta2 expression in the mature cerebellum of hotfoot mice promotes parallel fiber synaptogenesis and axonal competition. PLoS One. 2009;4(4):e5243.
Morando L et al. Role of glutamate delta −2 receptors in activity-dependent competition between heterologous afferent fibers. Proc Natl Acad Sci U S A. 2001;98(17):9954–9.
Bravin M et al. Control of spine formation by electrical activity in the adult rat cerebellum. Proc Natl Acad Sci U S A. 1999;96(4):1704–9.
Ekerot CF, Jorntell H. Parallel fiber receptive fields: a key to understanding cerebellar operation and learning. Cerebellum. 2003;2(2):101–9.
Sultan F, Bower JM. Quantitative Golgi study of the rat cerebellar molecular layer interneurons using principal component analysis. J Comp Neurol. 1998;393(3):353–73.
Jorntell H et al. Cerebellar molecular layer interneurons—computational properties and roles in learning. Trends Neurosci. 2010;33(11):524–32.
Gao W et al. Cerebellar cortical molecular layer inhibition is organized in parasagittal zones. J Neurosci. 2006;26(32):8377–87.
Montarolo PG, Palestini M, Strata P. The inhibitory effect of the olivocerebellar input on the cerebellar Purkinje cells in the rat. J Physiol. 1982;332:187–202.
Cerminara NL, Rawson JA. Evidence that climbing fibers control an intrinsic spike generator in cerebellar Purkinje cells. J Neurosci. 2004;24(19):4510–7.
Eccles JC, Ito M, Szentágothai J. The cerebellum as a neuronal machine. Berlin: Springer; 1967.
Ito M. The cerebellum and neural control. New York: Raven; 1984.
Dean P et al. The cerebellar microcircuit as an adaptive filter: experimental and computational evidence. Nat Rev Neurosci. 2010;11(1):30–43.
Marquez-Ruiz J, Cheron G. Sensory stimulation-dependent plasticity in the cerebellar cortex of alert mice. PLoS One. 2012;7(4):e36184.
Sakurai M. Synaptic modification of parallel fibre-Purkinje cell transmission in in vitro guinea-pig cerebellar slices. J Physiol. 1987;394:463–80.
Jorntell H, Ekerot CF. Receptive field remodeling induced by skin stimulation in cerebellar neurons in vivo. Front Neural Circ. 2011;5:3.
Badura A et al. Climbing fiber input shapes reciprocity of Purkinje cell firing. Neuron. 2013;78(4):700–13.
Brooks JX, Cullen KE. The primate cerebellum selectively encodes unexpected self-motion. Curr Biol. 2013;23(11):947–55.
Dean P et al. An adaptive filter model of cerebellar zone C3 as a basis for safe limb control? J Physiol. 2013;591(Pt 22):5459–74.
Bengtsson F, Jorntell H. Specific relationship between excitatory inputs and climbing fiber receptive fields in deep cerebellar nuclear neurons. PLoS One. 2014;9(1):e84616.
Zhou H et al. Cerebellar modules operate at different frequencies. Elife. 2014;3:e02536.
Xiao J et al. Systematic regional variations in Purkinje cell spiking patterns. PLoS One. 2014;9(8):e105633.
Ekerot CF, Larson B. Termination in overlapping sagittal zones in cerebellar anterior lobe of mossy and climbing fiber paths activated from dorsal funiculus. Exp Brain Res. 1980;38(2):163–72.
Sillitoe RV et al. Golgi cell dendrites are restricted by Purkinje cell stripe boundaries in the adult mouse cerebellar cortex. J Neurosci. 2008;28(11):2820–6.
Chadderton P, Margrie TW, Hausser M. Integration of quanta in cerebellar granule cells during sensory processing. Nature. 2004;428(6985):856–60.
Conflict of Interest
The author declares that he has no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jörntell, H. Cerebellar Synaptic Plasticity and the Credit Assignment Problem. Cerebellum 15, 104–111 (2016). https://doi.org/10.1007/s12311-014-0623-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12311-014-0623-y