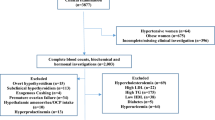
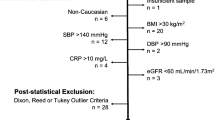
Abstract
Clinical reference intervals among Indian population are poorly defined. Therefore, there is an urgent need to establish local clinical laboratory reference intervals for healthy Indian population. The present study aimed to identify the 95 % reference interval for hematological and biochemical parameters in apparently healthy Indian population. We undertook a multicentric cross-sectional study conducted at Apollo Hospitals Educational and Research Foundation across India. Of which 10,665 reference individuals identified as healthy by physicians. The 95 % of the reference distribution was estimated using 2.5th and 97.5th percentile reference limits. The 95 % reference intervals for hemoglobin (Males: 12.3–17 g/dL; Females: 9.9–14.3 g/dL), platelet count (Males: 1.3–3.8; Females: 1.3–4.2 Lakhs/µL), erythrocyte sedimentation rate (Males: 2–22; Females: 4–55 mm/h), serum uric acid in males: 3.5–8.2 mg/dL, gamma glutamyl transferase (Males: 13–61 U/L), fasting blood glucose (Males: 78–110 mg/dL), total cholesterol (Males: 115–254 mg/dL), low density lipoprotein (Males: 60–176 mg/dL) and triglycerides (Males: 55–267 mg/dL, Females: 52–207 mg/dL) were different from currently used reference values. Additionally need for gender based partitioning were observed for triglycerides and gamma glutamyl transferase. The observed findings are of clinical significance and it needs to be validated with additional community based studies.
Similar content being viewed by others
References
Solberg HE. International Federation of Clinical Chemistry, expert panel on theory of reference values: approved recommendation (1986) on the theory of reference values. Part 1. The concept of reference values. Clin Chim Acta. 1987;165:111–8.
PetitClerc C, Solberg HE. International Federation of Clinical Chemistry, expert panel on theory of reference values: approved recommendation (1987) on the theory of reference values. Part 2. Selection of individuals for the production of reference values. J Clin Chem Clin Biochem. 1987;25:639–44.
Solberg HE, PetitClerc C. International Federation of Clinical Chemistry (IFCC), expert panel on theory of reference values: approved recommendation (1988) on the theory of reference values. Part 3. Preparation of individuals and collection of specimens for the production of reference values. J Clin Chem Clin Biochem. 1988;26:593–8.
Solberg HE, Stamm D. International Federation of Clinical Chemistry (IFCC) recommendation: the theory of reference values. Part 4. Control of analytical variation in the production, transfer and application of reference values. J Automat Chem. 1991;13:231–4.
Solberg HE. International Federation of Clinical Chemistry, expert panel on theory of reference values: approved recommendation: on the theory of reference values. Part 5. Statistical treatment of collected reference values: determination of reference limits. Clin Chim Acta. 1987;170:S13–32.
Dybkaer R, Solberg HE. International Federation of Clinical Chemistry (IFCC), expert panel on theory of reference values: approved recommendation (1987) on the theory of reference values. Part 6. Presentation of observed values related to reference values. Clin Chim Acta. 1987;170:S33–42.
National Committee for Clinical Laboratory Standards (NCCLS). How to define and determine reference intervals in the clinical laboratory; approved guideline, 2nd ed, NCCLS document C28-A2. Wayne, PA: NCCLA.
CLSI. Defining, establishing, and verifying reference intervals in the clinical laboratory; approved guideline, 3rd ed, CLSI document C28-A3. Wayne, PA: CLSI; 2008.
Yadav D, Mishra S, Gupta M, Sharma P. Reference intervals of certain liver specific biochemical analytes in Indian population. Indian J Clin Biochem. 2011;26:98–9.
Horn PS, Pesce AJ. Effect of ethnicity on reference intervals. Clin Chem. 2002;48:1802–4.
Dosoo DK, Kayan K, Adu-Gyasi D, Kwara E, Ocran J, Osei-Kwakye K, et al. Haematological and biochemical reference values for healthy adults in the middle belt of Ghana. PLoS ONE. 2012;7:e36308.
Johnson AM, Hyltoft Petersen P, Whicher JT, Carlstrom A, MacLennan S. Reference intervals for plasma proteins: similarities and differences between Caucasian and Asian Indian males in Yorkshire, UK. Clin Chem Lab Med. 2004;42:792–9.
Davis BH, Bigelow NC. Performance evaluation of a hematology blood counter with five-part leukocyte differential capability. Am Clin Lab. 1999;18:8–9.
Bain BJ. Ethnic and sex differences in the total and differential white cell count and platelet count. J Clin Pathol. 1996;49:664–6.
Azikiwe AN. Platelet count values in healthy Nigeria medical students in Jos. East Afr Med J. 1984;61:482–5.
Sundaram M, Mohanakrishnan J, Murugavel KG, Shankar EM, Solomon S, Srinivas CN, et al. Ethnic variation in certain hematological and biochemical reference intervals in a south Indian healthy adult population. Eur J Intern Med. 2008;19:46–50.
Yadav D, Mishra S, Gupta M, John PJ, Sharma P. Establishment of reference interval for liver specific biochemical parameters in apparently healthy north Indian population. Indian J Clin Biochem. 2013;28:30–7.
Das M, Saikia M. Estimation of reference interval of lipid profile in Assamese population. Indian J Clin Biochem. 2009;24:190–3.
Durgawale P, Patil S, Shukla PS, Sontakke A, Kakade S, Yadav S. Evaluation of reference intervals of serum lipid profile from healthy population in western Maharashtra. Indian J Clin Biochem. 2009;24:30–5.
Kaur V, Verma M, Kaur A, Gupta S, Singh K. To establish the reference intervals of lipid profile in Punjab. Indian J Clin Biochem. 2012;27:290–5.
Malati T, Mahesh MRU. To Reference intervals for serum total cholesterol, HDL-cholesterol, LDL-cholesterol, triglycerides, Lp (a), apolipoprotein A-I, A-II, B, C-II, C-III and E in healthy South Indians from Andhra Pradesh. Indian J Clin Biochem. 2009;24:343–55.
Furruqh S, Anitha D, Venkatesh T. Estimation of reference values in liver function test in health plan individuals of an urban south Indian population. Indian J Clin Biochem. 2004;19:72–9.
Verma M, Khadapkar R, Sahu PS, Das BR. Comparing age-wise reference intervals for serum creatinine concentration in a reality check of the recommended cut-off. Indian J Clin Biochem. 2006;21:90–4.
Baliarsingh S, Sharma N. Serum uric acid level is an indicator of total cholesterol and low density lipoprotein cholesterol in men below 45 years in age but not older males. Clin Lab. 2012;58:545–50.
Pasic MD, Colantonio DA, Chan MK, Venner AA, Brinc D, Adeli K. Influence of fasting and sample collection time on 38 biochemical markers in healthy children: a CALIPER substudy. Clin Biochem. 2012;45:1125–30.
Acknowledgments
We thank Prof. Ranjit Roy Chaudhury for his guidance and support. We also thank AHERF research team who helped for data collection and management. Our special thanks to Dr. Prathap C. Reddy, Ms. Shobana Kamineni and Mr. Sridhar for funding the project.
Conflict of Interest
The authors have declared that no competing interest exists.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sairam, S., Domalapalli, S., Muthu, S. et al. Hematological and Biochemical Parameters in Apparently Healthy Indian Population: Defining Reference Intervals. Ind J Clin Biochem 29, 290–297 (2014). https://doi.org/10.1007/s12291-013-0365-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12291-013-0365-5