Abstract
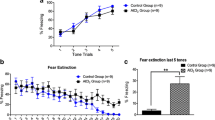
Aluminum is associated with etiology of many neurodegenerative diseases specially Alzheimer’s disease. Chronic exposure to aluminum via drinking water results in aluminum deposition in the brain that leads to cognitive deficits. The study aimed to determine the effects of aluminum on cholinergic biomarkers, i.e., acetylcholine level, free choline level, and choline acetyltransferase gene expression, and how cholinergic deficit affects novel object recognition and sociability in mice. Mice were treated with AlCl3 (250 mg/kg). Acetylcholine level, free choline level, and choline acetyltransferase gene expression were determined in cortex, hippocampus, and amygdala. The mice were subjected to behavior tests (novel object recognition and social novelty preference) to assess memory deficits. The acetylcholine level in cortex and hippocampus was significantly reduced in aluminum-treated animals, as compared to cortex and hippocampus of control animals. Acetylcholine level in amygdala of aluminum-treated animals remained unchanged. Free choline level in all the three brain parts was found unaltered in aluminum-treated mice. The novel object recognition memory was severely impaired in aluminum-treated mice, as compared to the control group. Similarly, animals treated with aluminum showed reduced sociability compared to the control mice group. Our study demonstrates that aluminum exposure via drinking water causes reduced acetylcholine synthesis in spite of normal free choline availability. This deficit is caused by reduced recycling of acetylcholine due to lower choline acetyltransferase level. This cholinergic hypofunction leads to cognitive and memory deficits. Moreover, hippocampus is the most affected brain part after aluminum intoxication.




Similar content being viewed by others
References
Abd-Elhady RM, Elsheikh AM, Khalifa AE (2013) Anti-amnestic properties of Ginkgo biloba extract on impaired memory function induced by aluminum in rats. Int J Dev Neurosci 31(7):598–607
Pohl HR, Roney N, Abadin HG (2011) Metal ions affecting the neurological system. Met Ions Life Sci 8(247):62
Zatta P, Ibn-Lkhayat-Idrissi M, Zambenedetti P, Kilyen M, Kiss T (2002) In vivo and in vitro effects of aluminum on the activity of mouse brain acetylcholinesterase. Brain Res Bull 59(1):41–45
Hu W-P, Li X-M, Chen J-G, Li Z-W (2007) Potentiation of the nicotinic acetylcholine receptor by aluminum in mammalian neurons. Neuroscience 149(1):1–6
Gulya K, Rakonczay Z, Kasa P (1990) Cholinotoxic effects of aluminum in rat brain. J Neurochem 54(3):1020–1026
Kaizer R, Correa M, Gris L, Da Rosa C, Bohrer D, Morsch V, Schetinger MRC (2008) Effect of long-term exposure to aluminum on the acetylcholinesterase activity in the central nervous system and erythrocytes. Neurochem Res 33(11):2294–2301
Brus R, Szkilnik R, Popieluch I, Kostrzewa RM, Mengel K (1997) Effect of aluminium exposure on central serotonine and muscarine receptors reactivity in rats. Med Sci Monit 3(5):BR631–BR636
Yellamma K, Saraswathamma S, Kumari BN (2010) Cholinergic system under aluminium toxicity in rat brain. Toxicol Int 17(2):106
Stevanović ID, Jovanović MD, Čolić M, Jelenković A, Bokonjić D, Ninković M (2010) Nitric oxide synthase inhibitors protect cholinergic neurons against AlCl 3 excitotoxicity in the rat brain. Brain Res Bull 81(6):641–646
Schliebs R, Arendt T (2006) The significance of the cholinergic system in the brain during aging and in Alzheimer’s disease. J Neural Transm 113(11):1625–1644
Fabian-Fine R, Skehel P, Errington ML, Davies HA, Sher E, Stewart MG, Fine A (2001) Ultrastructural distribution of the α7 nicotinic acetylcholine receptor subunit in rat hippocampus. J Neurosci 21(20):7993–8003
Mahboob A, Farhat SM, Iqbal G, Babar MM, Nabavi SM, Ahmed T (2016) Alpha-lipoic acid-mediated activation of muscarinic receptors improves hippocampus-and amygdala-dependent memory. Brain Res Bull 122:19–28
AN Hashmi, Yaqinuddin A, Ahmed T (2014) Pharmacological effects of Ibuprofen on learning and memory, muscarinic receptors genes expression and APP isoforms levels in pre-frontal cortex of AlCl3-induced toxicity mouse model. Int J Neurosci (0):1–37
Syed H, Ikram MF, Yaqinuddin A, Ahmed T (2015) Cyclooxygenase I and II inhibitors distinctly enhance hippocampal-and cortex-dependent cognitive functions in mice. Mol Med Rep 12(5):7649–7656
Wills MR, Savory J (1985) Water content of aluminum, dialysis dementia, and osteomalacia. Environ Health Perspect 63:141
Commissaris R, Cordon J, Sprague S, Keiser J, Mayor G, Rech R (1982) Behavioral changes in rats after chronic aluminum and parathyroid hormone administration. Neurobehav Toxicol Teratol 4(3):403
Golub MS, Donald JM, Gershwin ME, Keen CL (1989) Effects of aluminum ingestion on spontaneous motor activity of mice. Neurotoxicol Teratol 11(3):231–235
Iqbal G, Iqbal A, Mahboob A, Farhat S, Ahmed T (2016) Memory enhancing effect of black pepper in the AlCl3-induced neurotoxicity mouse model is mediated through its active component chavicine. Curr Pharm Biotechnol
Harkany T, Lengyel Z, Kasa P, Gulya K (1995) Chronic aluminum treatment results in aluminum deposits and affects Ml muscarinic receptors in rat brain. Neurobiology (Budapest, Hungary) 4(1–2):35–43
Peng J-HF, Xu Z-C, Xu Z-X, Parker JC, Friedlander ER, Tang J-P, Melethil S (1992) Aluminum-induced acute cholinergic neurotoxicity in rat. Mol Chem Neuropathol 17(1):79–89
Shafer TJ, Mundy WR, Tilson HA (1993) Aluminum decreases muscarinic, adrenergic, and metabotropic receptor-stimulated phosphoinositide hydrolysis in hippocampal and cortical slices from rat brain. Brain Res 629(1):133–140
Maheswari S, Venkatakrishna Murali R, Balaji R (2014) Aluminium induced cholinotoxicity in zebra fish brain—a sequel of oxidative stress. Int J Adv Res 2:322–335
Julka D, Sandhir R, Gill KD (1995) Altered cholinergic metabolism in rat CNS following aluminum exposure: implications on learning performance. J Neurochem 65(5):2157–2164
Kumar S (1998) Biphasic effect of aluminium on cholinergic enzyme of rat brain. Neurosci Lett 248(2):121–123
Mayeux R, Stern Y (2012) Epidemiology of Alzheimer disease. Cold Spring Harb Perspect Med 2(8):a006239
Cherroret G, Desor D, Hutin M, Burnel D, Capolaghi B, Lehr P (1996) Effects of aluminum chloride on normal and uremic adult male rats. Biol Trace Elem Res 54(1):43–53
Connor DJ, Jope RS, Harrell LE (1988) Chronic, oral aluminum administration to rats: cognition and cholinergic parameters. Pharmacol Biochem Behav 31(2):467–474
Zhang R, Zhang J, Fang L, Li X, Zhao Y, Shi W, An L (2014) Neuroprotective effects of sulforaphane on cholinergic neurons in mice with Alzheimer’s disease-like lesions. Int J Mol Sci 15(8):14396–14410
Woolf NJ (1991) Cholinergic systems in mammalian brain and spinal cord. Prog Neurobiol 37(6):475–524
Stevanović ID, Jovanovic M, Colic M, Jelenkovic A, Bokonjic D, Ninkovic M, Stojanovic I (2011) N-nitro-L-arginine methyl ester influence on aluminium toxicity in the brain. Folia Neuropathol 49(3):219–229
Jelenković A, Jovanović MD, Stevanović I, Petronijević N, Bokonjić D, Živković J, Igić R (2014) Influence of the green tea leaf extract on neurotoxicity of aluminium chloride in rats. Phytother Res 28(1):82–87
Bielarczyk H, Tomaszewicz M, Szutowicz A (1998) Effect of aluminum on acetyl-CoA and acetylcholine metabolism in nerve terminals. J Neurochem 70(3):1175–1181
Jankowska A, Madziar B, Tomaszewicz M, Szutowicz A (2000) Acute and chronic effects of aluminum on acetyl-CoA and acetylcholine metabolism in differentiated and nondifferentiated SN56 cholinergic cells. J Neurosci Res 62(4):615–622
Szutowicz A, Bielarczyk H, Kisielevski Y, Jankowska A, Madziar B, Tomaszewicz M (1998) Effects of aluminum and calcium on acetyl-CoA metabolism in rat brain mitochondria. J Neurochem 71:2447–2453
Antunes M, Biala G (2012) The novel object recognition memory: neurobiology, test procedure, and its modifications. Cogn Process 13(2):93–110
Kruk-Słomka M, Michalak A, Budzyńska B, Biała G (2014) A comparison of mecamylamine and bupropion effects on memory-related responses induced by nicotine and scopolamine in the novel object recognition test in mice. Pharmacol Rep 66(4):638–646
Silvers JM, Harrod SB, Mactutus CF, Booze RM (2007) Automation of the novel object recognition task for use in adolescent rats. J Neurosci Methods 166(1):99–103
Ennaceur A (2010) One-trial object recognition in rats and mice: methodological and theoretical issues. Behav Brain Res 215(2):244–254
Buckmaster CA, Eichenbaum H, Amaral DG, Suzuki WA, Rapp PR (2004) Entorhinal cortex lesions disrupt the relational organization of memory in monkeys. J Neurosci 24(44):9811–9825
Clark RE, Zola SM, Squire LR (2000) Impaired recognition memory in rats after damage to the hippocampus. J Neurosci 20(23):8853–8860
Isaev N, Stelmashook E, Genrikhs E, Oborina M, Kapkaeva M, Skulachev V (2015) Alzheimer’s disease: an exacerbation of senile phenoptosis. Biochem Mosc 80(12):1578–1581
Acknowledgments
We are thankful to the Atta-ur-Rahman School of Applied Biosciences, National University of Sciences and Technology, Islamabad, Pakistan for providing funding, support, and research facilities to carry out this research project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The behavior test experiments complied with the rulings of the Institute of Laboratory Animal Research, Division on Earth and Life Sciences, National Institute of Health, USA (Guide for the Care and Use of Laboratory Animals). The research protocol was approved by the Internal Review Board (IRB), Atta-ur-Rahman School of Applied Biosciences, National University of Sciences and Technology.
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
All applicable international, national, and institutional guidelines for the care and use of animals were followed. This article does not contain any studies with human participants performed by any of the authors.
Rights and permissions
About this article
Cite this article
Farhat, S.M., Mahboob, A., Iqbal, G. et al. Aluminum-Induced Cholinergic Deficits in Different Brain Parts and Its Implications on Sociability and Cognitive Functions in Mouse. Biol Trace Elem Res 177, 115–121 (2017). https://doi.org/10.1007/s12011-016-0856-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-016-0856-3