Abstract
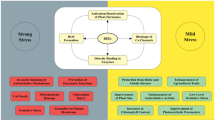
Rare earth elements, especially terbium (Tb), are high-valence heavy metal elements that accumulate in the environment, and they show toxic effects on plants. Signaling molecules regulate many physiological and biochemical processes in plants. How rare earth elements affect signaling molecules remains largely unknown. In the present study, the effects of Tb3+ on some extracellular and intracellular signaling molecules (gibberellic acid, abscisic acid, auxin, H2O2, and Ca2+) in horseradish leaves were investigated by using high-performance liquid chromatography, X-ray energy spectrometry, and transmission electron microscopy, and Tb3+ was sprayed on the surface of leaves. Tb3+ treatment decreased the auxin and gibberellic acid contents and increased the abscisic acid content. These changes in the contents of phytohormones (gibberellic acid, abscisic acid, and auxin) triggered excessive production of intracellular H2O2. Consequently, the increase in H2O2 content stimulated the influx of extracellular Ca2+ and the release of Ca2+ from Ca2+ stores, leading to Ca2+ overload and the resulting inhibition of physiological and biochemical processes. The effects outlined above were more evident with increasing the concentration of Tb3+ sprayed on horseradish leaves. Our data provide a possible underlying mechanism of Tb3+ action on plants.



Similar content being viewed by others
References
Redling K (2006) Rare earth elements in agriculture with emphasis on animal husbandry. Ludwig-Maximilians-Universität München, LMU München
Veitch NC (2004) Horseradish peroxidase: a modern view of a classic enzyme. Phytochemistry 65(3):249–259
Guo SF, Cao R, Lu AH, Zhou Q, Lu TH, Ding XL, Li CJ, Huang XH (2008) One of the possible mechanisms for the inhibition effect of Tb(III) on peroxidase activity in horseradish (Armoracia rusticana) treated with Tb(III). J Biol Inorg Chem 13(4):587–597
Wang LH, Zhou Q, Lu TH, Ding XL, Huang XH (2010) Molecular and cellular mechanism of effect of La(III) on horseradish peroxidase. J Biol Inorg Chem 15(7):1063–1069
Guo XS, Zhou Q, Lu TH, Fang M, Huang XH (2007) Distribution and translocation of 141Ce(III) in horseradish. Ann Bot 100(7):1459–1465
Wang LH, Zhou Q, Huang XH (2009) Photosynthetic responses to heavy metal terbium stress in horseradish leaves. Chemosphere 77(7):1019–1025
DalCorso G, Farinati S, Furini A (2010) Regulatory networks of cadmium stress in plants. Plant Signal Behav 5:663–667
Buchanan B, Gruissem W, Jones R (2000) Biochemistry & molecular biology of plants. Wiley, New York
Wang LH, Huang XH, Zhou Q (2008) Effects of rare earth elements on the distribution of mineral elements and heavy metals in horseradish. Chemosphere 73(3):314–319
Hu ZY, Richter H, Sparovek G, Schnug E (2004) Physiological and biochemical effects of rare earth elements on plants and their agricultural significance: a review. J Plant Nutr 27:183–220
Wang LH, Zhou Q, Huang XH (2010) Effects of heavy metal terbium on contents of cytosolic nutrient elements in horseradish cell. Ecotoxicol Environ Saf 73(5):1012–1017
Lichtenthaler H (1987) Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods Enzymol 148(2):350–382
Shah K, Kumar R, Verma S, Dubey R (2001) Effect of cadmium on lipid peroxidation, superoxide anion generation and activities of antioxidant enzymes in growing rice seedlings. Plant Sci 161:1135–1144
Hammerschmidt R, Nuckles E, Kug J (1982) Association of enhanced peroxidase activity with induced systemic resistance of cucumber to Colletotrichum lagenarium. Physiol Plant Pathol 20:77–82
Ma Z, Ge L, Lee ASY, Yong JWH, Tan SN, Ong ES (2008) Simultaneous analysis of different classes of phytohormones in coconut (Cocos nucifera L.) water using high-performance liquid chromatography and liquid chromatography–tandem mass spectrometry after solid-phase extraction. Anal Chim Acta 610(2):274–281
Bóka K, Orbán N, Kristóf Z (2007) Dynamics and localization of H2O2 production in elicited plant cells. Protoplasma 230:89–97
Pellinen R, Palva T, Kangasjarvi J (1999) Subcellular localization of ozone-induced hydrogen peroxide production in birch (Betula pendula) leaf cells. Plant J 20:349–356
Bestwick CS, Brown IR, Bennett MHR, Mansfield JW (1997) Localization of hydrogen peroxide accumulation during the hypersensitive reaction of lettuce cells to Pseudomonas syringae pv phaseolicola. Plant Cell 9(2):209–221
Slezak J, Tribulova N, Pristacova J, Uhrik B, Thomas T, Khaper N, Kaul N, Singal P (1995) Hydrogen peroxide changes in ischemic and reperfused heart. Cytochemistry and biochemical and X-ray microanalysis. Am J Pathol 147(3):772–781
Tian HQ, Kuang A, Musgrave ME, Russell SD (1998) Calcium distribution in fertile and sterile anthers of a photoperiod-sensitive genic male-sterile rice. Planta 204(2):183–192
Wang S, Tiwari S, Hagen G, Guilfoyle T (2005) Auxin response factor 7 restores the expression of auxin-responsive genes in mutant Arabidopsis leaf mesophyll protoplasts. Plant Cell 17(7):1979
Kovtun Y, Chiu WL, Sheen J (2000) Functional analysis of oxidative stress-activated mitogen-activated protein kinase cascade in plants. Proc Natl Acad Sci U S A 97:2940–2945
Minamiguchi K, Kumagai H, Masuda T, Kawada M, Ishizuka M, Takeuchi T (2001) Thiolutin, an inhibitor of HUVEC adhesion to vitronectin, reduces paxillin in HUVECs and suppresses tumor cell induced angiogenesis. Int J Cancer 93:307–316
Leung J, Giraudat J (1998) Abscisic acid signal transduction. Annu Rev Plant Biol 49:199–222
Fan L, Feng X, Wang Y, Deng X (2007) Gibberellin signal transduction in rice. J Integr Plant Biol 49:731
Macdonald H (1997) Auxin perception and signal transduction. Physiol Planta 100:423–430
Raven P, Evert R, Eichhorn S (2004) Biology of plants. WH Freeman, New York
He H, Serraj R, Yang Q (2009) Changes in OsXTH gene expression, ABA content, and peduncle elongation in rice subjected to drought at the reproductive stage. Acta Physiol Plant 31:749–756
Kutlu N, Terzi R, Tekeli C, Senel G, Battal P, Kadioglu A (2009) Changes in anatomical structure and levels of endogenous phytohormones during leaf rolling in Ctenanthe setosa under drought stress. Turk J Biol 33:115–122
Guan L, Zhao J, Scandalios J (2000) Cis-elements and trans-factors that regulate expression of the maize Cat 1 antioxidant gene in response to ABA and osmotic stress: H2O2 is the likely intermediary signaling molecule for the response. Plant J 22:87–95
Joo J, Bae Y, Lee J (2001) Role of auxin-induced reactive oxygen species in root gravitropism. Plant Physiol 126(3):1055–1060
Kwak JM, Nguyen V, Schroeder JI (2006) The role of reactive oxygen species in hormonal responses. Plant Physiol 141(2):323–329
Pei ZM, Murata Y, Benning G, Thomine S, Klusener B, Allen GJ, Grill E, Schroeder JI (2000) Calcium channels activated by hydrogen peroxide mediate abscisic acid signalling in guard cells. Nature 406(6797):731–734
Peters SC, Piper HM (2007) Reoxygenation-induced Ca2+ rise is mediated via Ca2+ influx and Ca2+ release from the endoplasmic reticulum in cardiac endothelial cells. Cardiovasc Res 73(1):164–171
Chen WP, Li PH (2001) Chilling-induced Ca2+ overload enhances production of active oxygen species in maize (Zea mays L.) cultured cells: the effect of abscisic acid treatment. Plant Cell Environ 24(8):791–800
Gasbarrini A, Borle AB, Farghali H, Bender C, Francavilla A, Vanthiel D (1992) Effect of anoxia on intracellular ATP, Na+(I), Ca2+(I), Mg2+(I), and cytotoxicity in rat hepatocytes. J Biol Chem 267(10):6654–6663
Hall KE, Browning MD, Dudek EM, Macdonald RL (1995) Enhancement of high-threshold calcium currents in rat primary afferent neurons by constitutively active protein-kinase-C. J Neurosci 15(9):6069–6076
Janeczek AH, Vanalten PJ, Reyes HM, Walter RJ (1993) Modulation of the cytoskeleton and intracellular calcium in leukocytes exhibiting a cancer-associated chemotaxis defect. J Leukoc Biol 54(4):351–359
Acknowledgments
The authors are grateful for the financial support of the National Natural Science Foundation of China (31170477, 21371100), the Foundation of the State Development and Reforming Committee (GFZ2071609), the Natural Science Foundation of Jiangsu Province (BK2011160, BK2009401), and Research and Innovation Project for Postgraduate of Higher Education Institutions of Jiangsu Province in 2012 (CXZZ12_0760).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Lihong Wang and Xianbo Zhang contributed equally to this work.
Rights and permissions
About this article
Cite this article
Wang, L., Zhang, X., Zhou, Q. et al. Effects of Terbium (III) on Signaling Molecules in Horseradish. Biol Trace Elem Res 164, 122–129 (2015). https://doi.org/10.1007/s12011-014-0209-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-014-0209-z