Abstract
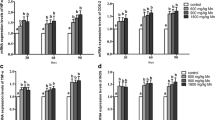
Manganese (Mn) is an essential element required for normal development and reproduction. However, little is known about the reproductive toxicity of Mn in birds. To investigate the Mn-induced toxicity on testicular trace element levels and crucial hormonal parameters on male reproduction in birds, 50-day-old male Hyline cocks were fed either a commercial diet or a Mn-supplemented diet. The changes in contents of copper (Cu), iron (Fe), zinc (Zn), and calcium (Ca) in testis were detected. Hormonal parameters were evaluated including the levels of testosterone (T), luteinizing hormone (LH), follicle-stimulating hormone (FSH), thyroid-stimulating hormone (TSH), triiodothyronine (T3), and thyroxine (T4) in the serum. The mRNA levels of luteinizing hormone receptor (LHR) and follicle-stimulating hormone receptor (FSHR) were determined in this study. The results showed that Mn was accumulated in testis, and the content of Cu, Fe, Zn, and Ca decreased. Exposure to Mn significantly lowered the content of T, LH, FSH, and the mRNA expression levels of LHR and FSHR. Levels of T3 and T4 appeared with a decreased tendency, and TSH presented no obvious regularity. It indicated that Mn exposure resulted in the disbalance of testicular trace elements and influenced hormone levels in the molecular level, which may be possible underlying reproductive toxicity mechanism induced by Mn.




Similar content being viewed by others
References
Aschner JL, Aschner M (2005) Nutritional aspects of manganese homeostasis. Mol Aspects Med 26:353–362
Takeda A (2003) Manganese action in brain function. Brain Res Rev 41:79–87
Xu XR, Li RB, Wang WH, Gu JD (2005) Decolorization of dyes and textile waste water by potassium permanganate. Chemosphere 59:893–898
Park RM, Bowler RM, Roels HA (2009) Exposure-response relationship and risk assessment for cognitive deficits in early welding-induced manganism. J Occup Environ Med 51:1125–1136
Furness RW, Camphuysen CJ (1997) Seabirds as monitors of the marine environment. ICES J Mar Sci 54:726–737
De Luca-Abbott SB, Wong BS, Peakall DB, Lam PK, Young L, Lam MH, Richardson BJ (2001) Review of effects of water pollution on the breeding success of waterbirds, with particular reference to Ardeids in Hong Kong. Ecotoxicology 10:327–349
Sakellarides TM, Konstantinou IK, Hela DG, Lambropoulou D, Dimou A, Albanis TA (2006) Accumulation profiles of persistent organochlorines in liver and fat tissues of various waterbird species from Creece. Chemosphere 63:1392–1409
Bro-Rasmussen F (1996) Contamination by persistent chemicals in food chain and human health. Sci Total Environ 188(suppl 1):S45–S60
Janssen DL, Dauwe T, Pinxten R, Bervoets L, Blust R, Eens M (2003) Effects of heavy metal exposure on the condition and health of nestling of the great tit (Parus major), a small songbirds species. Environ Pollut 126:267–274
Dauwe T, Janssens E, Kempenaers B, Eens M (2004) The effect of heavy metal exposure on egg size, eggshell thickness and the number of spermatozoa in blue tit Parus caerulens eggs. Environ Pollut 82:301–310
Liu XF, Li ZP, Han CR, Zhang ZW, Xu SW (2012) Effects of dietary manganese on Cu, Fe, Zn, Ca, IL-1β, and IL2 changes of immune organs in cocks. Biol Trace Elem Res 148:336–344
Mathur N, Pandey G, Jain GC (2010) Male reproductive toxicity of some selected metals: a review. J Biol Sci 10:396–404
Ponnapakkam TP, Sam GH, Iszard MB (2003) Histopathological changes in the testis of the Sprague Dawley rat following orally administered manganese. Bull Environ Contam Toxicol 71(6):1151–1157
Laskey JW, Rehnberg GL, Hein JF, Laws SC, Edens FW (1985) Assessment of the male reproductive system in the preweanling rat following Mn3O4 exposure. J Toxicol Environ Health 15:339–350
Cheng J, FU JL, Zhou ZC (2003) The inhibitory effects of manganese on steroidogenesis in rat primary Leydig cells by disrupting steroidogenic acute regulatory (StAR) protein expression. Toxicology 187(2–3):139–148
Rivera-Manci S, Rios C, Montes S (2011) Manganese accumulation in the CNS and associated pathologies. Biometals 24:811–825
Eybl V, Kotyzova D (2010) Protective effect of manganese in cadmium-induced hepatic oxidative damage, changes in cadmium distribution and trace elements level in mice. Interdiscip Toxicol 3(2):68–72
Lee B, Pine M, Johnson L, Rettori V, Hiney JK, Dees WL (2006) Manganese acts centrally to activate reproductive hormone secretion and pubertal development in male rats. Reprod Toxicol 22(4):580–585
Sogut O, Percin F (2011) Trace elements in the kidney tissue of bluefin tuna (Thunnus thynnus L.1758) Turkish seas. Afr J Biotechnol 10:1252–1259
AL-Gahri MA, Almussali MS (2008) Microelement contents of locally produced and imported wheat grains in Yemen. E-J Chem 5:838–843
Sun B, Wang R, Xu SW (2011) Dietary selenium affects selenoprotein W gene expression in the liver of chicken. Biol Trace Elem Res 143:1516–1523
Li JL, Li HX, Li S, Jiang ZH, Xu SW, Tang ZX (2011) Selenoprotein W gene expression in the gastrointestinal tract of chicken is affected by dietary selenium. Biometals 24:291–299
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29:e45
Ponnapakkam TP, Bailey KS, Graves KA, Iszard MB (2003) Assessment of male reproductive system in the CD-1 mice following oral manganese exposure. Reprod Toxicol 17:547–551
Wirth JJ, Rossano MG, Daly DC, Paly DC, Paneth N, Puscheck E, Potter RC, Diamond MP (2007) Ambient manganese exposure is negatively associated with human sperm motility and concentration. Epidemiology 18:270–273
Bauman DE, Currie WB (1980) Partitioning of nutrients during pregnancy and lactation: a review of mechanisms involving homeostasis and homeorhesis. J Dairy Sci 63:1514–1529
Capcarova M, Kolesarova A (2012) Trace elements in follicular fluid and their effects on reproductive functions. JMBFS 1:1039–1044
Zhang JH, Yang HB, Gu XL, Chen L, Li HQ, Wang JD (2008) Changes in zinc, iron, copper contents in testis and epididymis of rats exposure to sodium fluoride and sulfur dioxide. J Herb Med Toxicol 2:1–5
Kim H, Son HY, Bailey SM, Lee J (2009) Deletion of hepatic Ctr1 reveals its function in copper acquisition and compensatory mechanisms for copper homeostasis. Am J Physiol Gastrointest Liver Physiol 296:356–364
Khandelwal S, Ashguin M, Tandon SK (1984) Influence of essential elements on manganese intoxication. Bull Environ Contam Toxicol 32:10–19
EI-Seweidy M, Asker M, Ali S, Atteia H (2010) Effect of prolonged intake of iron enriched diet on testicular functions of experimental rats. Nat Sci 2:551–556
Zheng W, Zhao QQ, Slavkovich V, Aschner M, Graziano JH (1999) Alteration of iron homeostasis following chronic exposure to manganese in rats. Brain Res 833:125–132
Truong-Tran AQ, Ho LH, Chai F, Zalewski PD (2000) Cellular zinc fluxes and the regulation of apoptosis/gene-directed cell death. J Nutr 130:1459S–1466S
Saxena R, Bedwal RS, Mathur RS (1993) Histopathology of the testes of zinc deficient albino rats. Trace Elem Med 10:177–180
Zhang BY, Chen S, Ye FL (2002) Effect of manganese on heat stress protein synthesis of new-born rats. World J Gastroenterol 8:114–118
Dawson RD, Bidwell MT (2005) Dietary calcium limits size and growth of nestling tree swallows Tachycineta bicolor in a non-acidified landscape. J Avian Biol 36:127–134
Lu CL, Guo SC, Chen WP, Kuang XC (2006) Effect of manganese on cytosolic free calcium concentration in cortical neurons (China). Zhonghua Lao Dong Wei Sheng Zhi Ye Za Zhi 24(10):594–596
Gavin CE, Gunter K, Gunter TE (1999) Manganeses and calcium transport in mitochondria: implications for manganese toxicity. Neutotoxicology 20:445–453
Gunter TE, Miller LM, Gavin CE, Eliseev R (2004) Determination of the oxidation states of manganese in brain, liver, and heart mitochondria. J Neurochem 88:266–280
Aycan Z, Ocal G, Berberoglu M, Evliyaoglu O, Adiyaman P (2004) Monitoring serum testosterone levels on androgen therapy with long-acting testosterone esters in the prepubertal and pubertal age groups. Turk J Endocrinol Metab 3:113–116
Calderon J, Ortiz-Perez D, Yanez L, Diaz-Barriga F (2003) Human exposure to metals. Pathways of exposure, biomarkers of effect, and host factors. Ecotoxicol Environ Saf 56:93–103
Levavi-Sivan B, Bogerd J, Mananos EL, Gomez A, Lareyre JJ (2010) Perspectives on fish gonadotropins and their receptors. Gen Comp Endocrinol 165:412–437
Garcia-Lopez A, Jonge DH, Nobrega RH, Waal PP, Dijk V, Hemrika W, Taranger GL, Bogerd J, Schulz RW (2010) Studies in zebrafish reveal unusual cellular expression patterns of gonadotropin receptor messenger ribonucleic acids in the testis and unexpected functional differentiation of the gonadotropins. Endocrinology 151:2349–2360
Mclachlan RI, Wreford NG, O Donnell L, de Kretser DM, Robertson DM (1996) The endocrine regulation of spermatogeneses: independent roles for testosterone and FSH. J Endocrinol 14:1–9
Vischer HF, Granneman JC, Linskens MH, Schulz RW, Bogerd J (2003) Both recombinant African catfish LH and FSH are able to activate the African catfish FSH receptor. J Mol Endocrinol 31:133–140
Maruska KP, Fernald RD (2011) Plasticity of the reproductive axis caused by social status change in an African cichlid fish: II. Testicular gene expression and spermatogenesis. Endocrinology 152:291–302
Raja KP, Lakshmana SP, Aswani KK, Somasekhara RK (2010) Studies on the thyroid profile (T3, T4 and TSH) in estrous, cyclic and anoestrous buffaloes. Buffalo Bull 29:115–128
Sharma MK, Parchwani D, Maheria P, Upadhyah A (2012) Relationship between thyroid profile and semen quality. Natl J Community Med 3:20–24
Acknowledgments
This study was supported by the National Natural Science Foundation of China (30871902), Youth Science Foundation of Heilongjiang Province (QC2010026), and Scientific Research Foundation for Doctor of Harbin University of Commerce (12DL014).
Author information
Authors and Affiliations
Corresponding authors
Additional information
X. Liu and L. Zhang contributed equally to this work.
All other authors have read the manuscript and have agreed to submit it in its current form for consideration for publication in the journal.
Rights and permissions
About this article
Cite this article
Liu, Xf., Zhang, Lm., Zhang, Z. et al. Manganese-Induced Effects on Testicular Trace Element Levels and Crucial Hormonal Parameters of Hyline Cocks. Biol Trace Elem Res 151, 217–224 (2013). https://doi.org/10.1007/s12011-012-9549-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-012-9549-8