Abstract
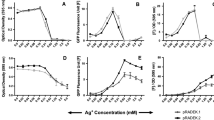
Fluorescent proteins from marine organisms represent potential candidates for biosensor development. In this paper, we described the isolation of a native green fluorescent protein from Anemonia sulcata and the cloning and purification of its equivalent as a recombinant protein in Escherichia coli. Furthermore, the spectroscopic behaviours of the native and recombinant GFPs were investigated as a function of Cu2+, Cd2+, Pb2+ and Ni2+ concentration. Our results suggest the high selectivity of both proteins at copper than the other metals and, for the recombinant protein, a great sensitivity at a very low concentration (0.1–1 μM). Moreover, starting from these data, using the combination of molecular biology techniques and optical setup, we developed a device for the detection of Cu2+ in water solutions. The quenching effect detected with the device showed that the relative attenuation of the signal (0.46 ± 0.02 AU) was slightly larger than the data measured by fluorescence spectra (0.65 ± 0.03 AU). The good sensitivity in the span of two orders of the magnitude of Cu2+ concentration, the fact that the instrument is made up of low-cost and sturdy parts and the selective quenching of rAsGFP to copper ions make this setup suited as a low cost, on-the-field, copper ion-specific biosensor.






Similar content being viewed by others
References
Trautwein, A. X. (1997). Bioinorganic chemistry: transition metals in biology and their coordination chemistry. Wiley-VCH, Weinheim.
Becker, J. S., Zoriy, M. V., Pickhardt, C., Palomero-Gallagher, N., & Zilles, K. (2005). Imaging of copper, zinc, and other elements in thin section of human brain samples (hippocampus) by laser ablation inductively coupled plasma mass spectrometry. Analytical chemistry, 77, 3208–3216.
Rao, G. P., Seshaiah, K., Rao, Y. K., & Wang, M. C. (2006). Solid phase extraction of Cd, Cu, and Ni from leafy vegetables and plant leaves using amberlite XAD-2 functionalized with 2-hydroxy-acetophenone-thiosemicarbazone (HAPTSC) and determination by inductively coupled plasma atomic emission spectroscopy. Journal of agricultural and food chemistry, 54, 2868–2872.
Xiang, Y., Li, Z., Chen, X., & Tong, A. (2008). Highly sensitive and selective optical chemosensor for determination of Cu2+ in aqueous solution. Talanta, 74, 1148–1153.
Bozkurt, S. S., & Cavas, L. (2009). Can Hg(II) be determined via quenching of the emission of green fluorescent protein from Anemonia sulcata var. smaragdina? Applied biochemistry and biotechnology, 158, 51–58.
Chapleau, R. R., Blomberg, R., Ford, P. C., & Sagermann, M. (2008). Design of a highly specific and noninvasive biosensor suitable for real-time in vivo imaging of mercury(II) uptake. Protein science: a publication of the Protein Society, 17, 614–622.
Eli, P., & Chakrabartty, A. (2006). Variants of DsRed fluorescent protein: development of a copper sensor. Protein science: a publication of the Protein Society, 15, 2442–2447.
Isarankura-Na-Ayudhya, C., Tantimongcolwat, T., Galla, H. J., & Prachayasittikul, V. (2010). Fluorescent protein-based optical biosensor for copper ion quantitation. Biological trace element research, 134, 352–363.
Rahimi, Y., Goulding, A., Shrestha, S., Mirpuri, S., & Deo, S. K. (2008). Mechanism of copper induced fluorescence quenching of red fluorescent protein, DsRed. Biochemical and biophysical research communications, 370, 57–61.
Richmond, T. A., Takahashi, T. T., Shimkhada, R., & Bernsdorf, J. (2000). Engineered metal binding sites on green fluorescence protein. Biochemical and biophysical research communications, 268, 462–465.
Sumner, J. P., Westerberg, N. M., Stoddard, A. K., Hurst, T. K., Cramer, M., Thompson, R. B., et al. (2006). DsRed as a highly sensitive, selective, and reversible fluorescence-based biosensor for both Cu(+) and Cu(2+) ions. Biosensors & bioelectronics, 21, 1302–1308.
Tansila, N., Becker, K., Isarankura Na-Ayudhya, C., Prachayasittikul, V., & Bulow, L. (2008). Metal ion accessibility of histidine-modified superfolder green fluorescent protein expressed in Escherichia coli. Biotechnology letters, 30, 1391–1396.
Balint, E. E., Petres, J., Szabo, M., Orban, C. K., Szilagyi, L., & Abraham, B. (2013). Fluorescence of a histidine-modified enhanced green fluorescent protein (EGFP) effectively quenched by copper(II) ions. Journal of fluorescence, 23, 273–281.
Fradkov, A. F., Verkhusha, V. V., Staroverov, D. B., Bulina, M. E., Yanushevich, Y. G., Martynov, V. I., et al. (2002). Far-red fluorescent tag for protein labelling. The Biochemical journal, 368, 17–21.
Peternel, S., Gaberc-Porekar, V., & Komel, R. (2009). Bacterial growth conditions affect quality of GFP expressed inside inclusion bodies. Acta Chimica Slovenica, 56, 860–867.
Fersht, A. (1999). Structure and mechanism in protein science: a guide to enzyme catalysis and protein folding. New York: Freeman.
Baird, G. S., Zacharias, D. A., & Tsien, R. Y. (2000). Biochemistry, mutagenesis, and oligomerization of DsRed, a red fluorescent protein from coral. Proceedings of the National Academy of Sciences of the United States of America, 97, 11984–11989.
Mizuno, H., Sawano, A., Eli, P., Hama, H., & Miyawaki, A. (2001). Red fluorescent protein from Discosoma as a fusion tag and a partner for fluorescence resonance energy transfer. Biochemistry, 40, 2502–2510.
Shagin, D. A., Barsova, E. V., Yanushevich, Y. G., Fradkov, A. F., Lukyanov, K. A., Labas, Y. A., et al. (2004). GFP-like proteins as ubiquitous Metazoan superfamily: evolution of functional features and structural complexity. Molecular and Biological Evolution, 21, 841–850.
Ward, W. W. (1998) Green fluorescent protein: properties, applications, and protocols. In: Chalfie M & Kain S (Eds). Wiley, New York, pp 45–75.
Wiedenmann, J., Elke, C., Spindler, K. D., & Funke, W. (2000). Cracks in the beta-can: fluorescent proteins from Anemonia sulcata (Anthozoa, Actinaria). Proceedings of the National Academy of Sciences of the United States of America, 97, 14091–14096.
Wiedenmann, J., Schenk, A., Rocker, C., Girod, A., Spindler, K. D., & Nienhaus, G. U. (2002). A far-red fluorescent protein with fast maturation and reduced oligomerization tendency from Entacmaea quadricolor (Anthozoa, Actinaria). Proceedings of the National Academy of Sciences of the United States of America, 99, 11646–11651.
Ormo, M., Cubitt, A. B., Kallio, K., Gross, L. A., Tsien, R. Y., & Remington, S. J. (1996). Crystal structure of the Aequorea victoria green fluorescent protein. Science, 273, 1392–1395.
Sniegowski, J. A., Lappe, J. W., Patel, H. N., Huffman, H. A., & Wachter, R. M. (2005). Base catalysis of chromophore formation in Arg96 and Glu222 variants of green fluorescent protein. The Journal of biological chemistry, 280, 26248–26255.
Wood, T. I., Barondeau, D. P., Hitomi, C., Kassmann, C. J., Tainer, J. A., & Getzoff, E. D. (2005). Defining the role of arginine 96 in green fluorescent protein fluorophore biosynthesis. Biochemistry, 44, 16211–16220.
Zagranichny, V. E., Rudenko, N. V., Gorokhovatsky, A. Y., Zakharov, M. V., Balashova, T. A., & Arseniev, A. S. (2004). Traditional GFP-type cyclization and unexpected fragmentation site in a purple chromoprotein from Anemonia sulcata, asFP595. Biochemistry, 43, 13598–13603.
Regan, L. (1993). The design of metal-binding sites in proteins. Annual review of biophysics and biomolecular structure, 22, 257–287.
Walter, E. D., Chattopadhyay, M., & Millhauser, G. L. (2006). The affinity of copper binding to the prion protein octarepeat domain: evidence for negative cooperativity. Biochemistry, 45, 13083–13092.
Navarra, G., Leone, M., & Militello, V. (2007). Thermal aggregation of beta-lactoglobulin in presence of metal ions. Biophysical chemistry, 131, 52–61.
Navarra, G., Giacomazza, D., Leone, M., Librizzi, F., Militello, V., & San Biagio, P. L. (2009). Thermal aggregation and ion-induced cold-gelation of bovine serum albumin. European biophysics journal : EBJ, 38, 437–446.
Vogt, A., D’angelo, C., Oswald, F., Denzel, A., Mazel, C. H., Matz, M. V., et al. (2008). A green fluorescent protein with photoswitchable emission from the deep sea. PloS one, 3, e3766.
Sigel, H. (1982) Metal ions in biological systems, properties of copper, vol. 12. Marcel Dekker, New York.
Silvia, J., & Williams, R. (1991). The biological chemistry of the elements: the inorganic chemistry of life. Oxford: Claredon.
Fitzgerald, D. J. (1998). Safety guidelines for copper in water. The American journal of clinical nutrition, 67, 1098S–1102S.
Acknowledgments
We thank Dr. Salvatore Mazzola for critical reading of the manuscript and Dr. Aldo Nicosia and Dr. Monica Salamone for helpful discussion. This work was supported by PO FERS 2007/2013 Linea di intervento 4.1.1.2.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Masullo, T., Puccio, R., Di Pierro, M. et al. Development of a Biosensor for Copper Detection in Aqueous Solutions Using an Anemonia sulcata Recombinant GFP. Appl Biochem Biotechnol 172, 2175–2187 (2014). https://doi.org/10.1007/s12010-013-0669-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-013-0669-1