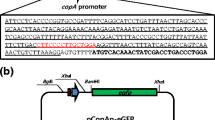
Abstract
The anthropogenic release of toxic metals into the environment poses danger to the health of both humans and the local ecosystem. Biosensors for the detection of metals have been developed to improve our ability to monitor these environmental contaminants, yet most of these sensors use heterotrophic bacterial hosts, which require a fixed carbon source and do not typically grow in natural waterways. In this study, we constructed and characterized metal sensors for development of a photoautotrophic biosensor using Synechococcus sp. PCC 7002. We characterized gold and copper sensors based on modified MerR transcriptional activators: GolSA113T, with improved gold binding, and GolSCL, containing the metal-binding loop from CueR which binds both gold and copper. The metal-sensing constructs were first optimized and characterized in Escherichia coli MG1655. The addition of a strong ribosome binding site to the optical reporter protein increased translation of the fluorescent reporter, and expression of golSA113T from the rbc promoter of Synechococcus sp. PCC 7002 improved the response to gold in MG1655. In rich medium, the GolSA113T-based E. coli sensor detected gold at concentrations as low as 100 nM, while the GolSCL-based E. coli sensor detected gold and copper at sensitivities of 100 nM and 10 μM, respectively. Both E. coli sensors responded to gold and copper yet showed no detectable response to other metals. Abiotic factors, such as medium complexity, were found to influence the response of the E. coli sensors, with minimal medium resulting in higher sensitivities of detection. Expression of the GolSA113T- and GolSCL-based sensor constructs in the cyanobacterium Synechococcus sp. PCC 7002 resulted in photoautotrophic gold sensors, but these biosensors failed to produce a significant response to copper. Moreover, the fluorescence response of the cyanobacterial sensors to gold was significantly reduced compared to that of analogous E. coli sensors. While this effort demonstrates feasibility for the development of photoautotrophic biosensors, additional efforts to optimize sensor performance will be required.






Similar content being viewed by others
References
Bereza-Malcolm L, Aracic S, Franks AE (2016) Development and application of a synthetically-derived lead biosensor construct for use in gram-negative bacteria. Sensors 16(12):2174
Bergsland KJ, Haselkorn R (1991) Evolutionary relationships among eubacteria, cyanobacteria, and chloroplasts: evidence from the rpoC1 gene of Anabaena sp. strain PCC 7120. J Bacteriol 173(11):3446–3455. https://doi.org/10.1128/jb.173.11.3446-3455.1991
Bondarenko O, Rõlova T, Kahru A, Ivask A (2008) Bioavailability of Cd, Zn and Hg in soil to nine recombinant luminescent metal sensor bacteria. Sensors 8(11):6899–6923
Brayner R, Couté A, Livage J, Perrette C, Sicard C (2011) Micro-algal biosensors. Anal Bioanal Chem 401(2):581–597
Brocklehurst KR, Hobman JL, Lawley B, Blank L, Marshall SJ, Brown NL, Morby AP (1999) ZntR is a Zn (II)-responsive MerR-like transcriptional regulator of zntA in Escherichia coli. Mol Microbiol 31(3):893–902
Brown NL, Stoyanov JV, Kidd SP, Hobman JL (2003) The MerR family of transcriptional regulators. FEMS Microbiol Rev 27(2–3):145–163
Cerminati S, Soncini FC, Checa SK (2011) Selective detection of gold using genetically engineered bacterial reporters. Biotechnol Bioeng 108(11):2553–2560
Cerminati S, Soncini FC, Checa SK (2015) A sensitive whole-cell biosensor for the simultaneous detection of a broad-spectrum of toxic heavy metal ions. Chem Commun 51(27):5917–5920
Charrier T, Durand M-J, Jouanneau S, Dion M, Pernetti M, Poncelet D, Thouand G (2011) A multi-channel bioluminescent bacterial biosensor for the on-line detection of metals and toxicity. Part I: design and optimization of bioluminescent bacterial strains. Anal Bioanal Chem 400(4):1051–1060
Checa SK, Soncini FC (2011) Bacterial gold sensing and resistance. BioMetals 24(3):419–427
Checa SK, Espariz M, Audero MEP, Botta PE, Spinelli SV, Soncini FC (2007) Bacterial sensing of and resistance to gold salts. Mol Microbiol 63(5):1307–1318
Corbisier P, van der Lelie D, Borremans B, Provoost A, de Lorenzo V, Brown NL, Lloyd JR, Hobman JL, Csöregi E, Johansson G (1999) Whole cell-and protein-based biosensors for the detection of bioavailable heavy metals in environmental samples. Anal Chim Acta 387(3):235–244
Erbe J, Adams A, Taylor K, Hall L (1996) Cyanobacteria carrying an smt-lux transcriptional fusion as biosensors for the detection of heavy metal cations. J Ind Microbiol 17(2):80–83
Espah Borujeni A, Channarasappa AS, Salis HM (2014) Translation rate is controlled by coupled trade-offs between site accessibility, selective RNA unfolding and sliding at upstream standby sites. Nucleic Acids Res 42(4):2646–2659. https://doi.org/10.1093/nar/gkt1139
Griese M, Lange C, Soppa J (2011) Ploidy in cyanobacteria. FEMS Microbiol Lett 323(2):124–131
Hakkila K, Green T, Leskinen P, Ivask A, Marks R, Virta M (2004) Detection of bioavailable heavy metals in EILATox-Oregon samples using whole-cell luminescent bacterial sensors in suspension or immobilized onto fibre-optic tips. J Appl Toxicol 24(5):333–342
Hansen LH, Sørensen SJ (2000) Versatile biosensor vectors for detection and quantification of mercury. FEMS Microbiol Lett 193(1):123–127
Huang H-H, Lindblad P (2013) Wide-dynamic-range promoters engineered for cyanobacteria. J Biol Eng 7(1):10
Hurdebise Q, Tarayre C, Fischer C, Colinet G, Hiligsmann S, Delvigne F (2015) Determination of zinc, cadmium and lead bioavailability in contaminated soils at the single-cell level by a combination of whole-cell biosensors and flow cytometry. Sensors 15(4):8981–8999
Ibanez MM, Cerminati S, Checa SK, Soncini FC (2013) Dissecting the metal selectivity of MerR monovalent metal ion sensors in Salmonella. J Bacteriol 195(13):3084–3092. https://doi.org/10.1128/JB.00153-13
Ibanez MM, Checa SK, Soncini FC (2015) A single serine residue determines selectivity to monovalent metal ions in metalloregulators of the MerR family. J Bacteriol 197(9):1606–1613. https://doi.org/10.1128/JB.02565-14
Ivask A, Virta M, Kahru A (2002) Construction and use of specific luminescent recombinant bacterial sensors for the assessment of bioavailable fraction of cadmium, zinc, mercury and chromium in the soil. Soil Biol Biochem 34(10):1439–1447
Ivask A, Rõlova T, Kahru A (2009) A suite of recombinant luminescent bacterial strains for the quantification of bioavailable heavy metals and toxicity testing. BMC Biotechnol 9(1):41
Kang Y, Lee W, Kim S, Jang G, Kim B-G, Yoon Y (2018) Enhancing the copper-sensing capability of Escherichia coli-based whole-cell bioreporters by genetic engineering. Appl Microbiol Biotechnol 102(3):1513–1521
Karlsson J-O, Ostwald K, Kabjorn C, Andersson M (1994) A method for protein assay in Laemmli buffer. Anal Biochem 219(1):144–146
Khan S, Brocklehurst KR, Jones GW, Morby AP (2002) The functional analysis of directed amino-acid alterations in ZntR from Escherichia coli. Biochem Biophys Res Commun 299(3):438–445
Li P-S, Peng Z-W, Su J, Tao H-C (2014) Construction and optimization of a Pseudomonas putida whole-cell bioreporter for detection of bioavailable copper. Biotechnol Lett 36(4):761–766
López-Maury L, Giner-Lamia J, Florencio FJ (2012) Redox control of copper homeostasis in cyanobacteria. Plant Signal Behav 7(12):1712–1714
Ludwig M, Bryant DA (2012) Synechococcus sp. strain PCC 7002 transcriptome: acclimation to temperature, salinity, oxidative stress, and mixotrophic growth conditions. Front Microbiol 3:354
Markley AL, Begemann MB, Clarke RE, Gordon GC, Pfleger BF (2014) Synthetic biology toolbox for controlling gene expression in the cyanobacterium Synechococcus sp. strain PCC 7002. ACS Synth Biol 4(5):595–603
Miao AJ, Wang WX, Juneau P (2005) Comparison of Cd, Cu, and Zn toxic effects on four marine phytoplankton by pulse-amplitude-modulated fluorometry. Environ Toxicol Chem 24(10):2603–2611
Priyadarshi H, Alam A, Gireesh-Babu P, Das R, Kishore P, Kumar S, Chaudhari A (2012) A GFP-based bacterial biosensor with chromosomally integrated sensing cassette for quantitative detection of Hg (II) in environment. J Environ Sci 24(5):963–968
Riether K, Dollard M-A, Billard P (2001) Assessment of heavy metal bioavailability using Escherichia coli zntAp:: lux and copAp:: lux-based biosensors. Appl Microbiol Biotechnol 57(5–6):712–716
Rodriguez-Freire L, Avasarala S, Ali A-MS, Agnew D, Hoover JH, Artyushkova K, Latta DE, Peterson EJ, Lewis J, Crossey LJ (2016) Post Gold King Mine spill investigation of metal stability in water and sediments of the Animas River watershed. Environ Sci Technol 50(21):11539–11548
Ruffing AM (2013) Borrowing genes from Chlamydomonas reinhardtii for free fatty acid production in engineered cyanobacteria. J Appl Phycol 25(5):1495–1507
Ruffing AM (2014) Improved free fatty acid production in cyanobacteria with Synechococcus sp. PCC 7002 as host. Front Bioeng Biotechnol 2:17
Ruffing AM, Jensen TJ, Strickland LM (2016) Genetic tools for advancement of Synechococcus sp. PCC 7002 as a cyanobacterial chassis. Microb Cell Factories 15(1):190
Salis HM, Mirsky EA, Voigt CA (2009) Automated design of synthetic ribosome binding sites to control protein expression. Nat Biotechnol 27:946–950. https://doi.org/10.1038/nbt.1568
Sambrook J, Russell D, Maniatis T (2001) Molecular cloning, vol 1-3. Cold Spring Habour Laboratory Press, New York
Schmittgen TD, Livak KJ (2008) Analyzing real-time PCR data by the comparative CT method. Nat Protoc 3(6):1101–1108
Schneider GJ, Hasekorn R (1988) RNA polymerase subunit homology among cyanobacteria, other eubacteria and archaebacteria. J Bacteriol 170(9):4136–4140. https://doi.org/10.1128/jb.170.9.4136-4140.1988
Sridhar S, Steele-Mortimer O (2016) Inherent variability of growth media impacts the ability of Salmonella typhimurium to interact with host cells. PLoS One 11(6):e0157043. https://doi.org/10.1371/journal.pone.0157043
Stevens SE, Porter RD (1980) Transformation in Agmenellum quadruplicatum. Proc Natl Acad Sci U S A 77(10):6052–6056
Stevens S, Patterson C, Myers J (1973) The production of hydrogen peroxide by blue-green algae: a survey. J Phycol 9(4):427–430
Stoyanov JV, Brown NL (2003) The Escherichia coli copper-responsive copA promoter is activated by gold. J Biol Chem 278(3):1407–1410. https://doi.org/10.1074/jbc.C200580200
Stoyanov JV, Hobman JL, Brown NL (2001) CueR (YbbI) of Escherichia coli is a MerR family regulator controlling expression of the copper exporter CopA. Mol Microbiol 39(2):502–512
Stoyanov JV, Magnani D, Solioz M (2003) Measurement of cytoplasmic copper, silver, and gold with a lux biosensor shows copper and silver, but not gold, efflux by the CopA ATPase of Escherichia coli. FEBS Lett 546(2–3):391–394
Tseng H-W, Tsai Y-J, Yen J-H, Chen P-H, Yeh Y-C (2014) A fluorescence-based microbial sensor for the selective detection of gold. Chem Commun 50(14):1735–1737
Virta M, Lampinen J, Karp M (1995) A luminescence-based mercury biosensor. Anal Chem 67(3):667–669
Viveiros M, Dupont M, Rodrigues L, Couto I, Davin-Regli A, Martins M, Pagès J-M, Amaral L (2007) Antibiotic stress, genetic response and altered permeability of E. coli. PLoS One 2(4):e365. https://doi.org/10.1371/journal.pone.0000365
Xie WQ, Jäger K, Potts M (1989) Cyanobacterial RNA polymerase genes rpoC1 and rpoC2 correspond to rpoC of Escherichia coli. J Bacteriol 171(4):1967–1973. https://doi.org/10.1128/jb.171.4.1967-1973.1989
Zammit CM, Quaranta D, Gibson S, Zaitouna AJ, Ta C, Brugger J, Lai RY, Grass G, Reith F (2013) A whole-cell biosensor for the detection of gold. PLoS One 8(8):e69292
Acknowledgments
This work was supported by the Laboratory Directed Research and Development program at Sandia National Laboratories. Sandia National Laboratories is a multimission laboratory managed and operated by National Technology & Engineering Solutions of Sandia, LLC, a wholly owned subsidiary of Honeywell International, Inc., for the U.S. Department of Energy’s National Nuclear Security Administration under contract DE-NA0003525. RFL was supported by an appointment to the Intelligence Community Postdoctoral Research Fellowship Program at Sandia National Laboratories, administered by Oak Ridge Institute for Science and Education through an interagency agreement between the U.S. Department of Energy and Office of the Director of National Intelligence. The authors also acknowledge Dr. Bryan Carson (Sandia National Laboratories) for his generosity in sharing equipment.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical statement
This article does not contain any studies with human participants or animals performed by any of the authors.
Disclaimer
This paper describes objective technical results and analysis. Any subjective views or opinions that might be expressed in the paper do not necessarily represent the views of the U.S. Department of Energy or the United States Government.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 2471 kb)
Rights and permissions
About this article
Cite this article
Lacey, R.F., Ye, D. & Ruffing, A.M. Engineering and characterization of copper and gold sensors in Escherichia coli and Synechococcus sp. PCC 7002. Appl Microbiol Biotechnol 103, 2797–2808 (2019). https://doi.org/10.1007/s00253-018-9490-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-018-9490-7