Abstract
Dengue is the fastest growing mosquito-borne disease worldwide, causing nearly 400 million infections annually. A universally applicable dengue virus vaccine is required to arrest its spread. Here, we generated an edible dengue vaccine by expressing the dengue fusion protein in tomatoes, which is a desirable expression system owing to the inherent adjuvanticity of alpha tomatine and immunogenicity of the tomato lectin/microbial antigen complex. The B subunit of Vibrio cholera toxin (CTB) was genetically fused to dengue envelope antigen for improved delivery to antigen-presenting cells and enhanced immunogenicity, while avoiding immunological tolerance. We utilized domain III of the dengue envelope protein (EDIII), as it has been shown to induce serotype-specific neutralizing antibodies. The CTB–EDIII fusion gene construct containing an endoplasmic reticulum target sequence was introduced into tomato plants by Agrobacterium tumefaciens-mediated gene transformation, and the expression of CTB–EDIII in transgenic plants was confirmed by DNA, RNA and protein analyses. Accumulated fusion protein accounted for up to 0.015 % of total soluble protein, and it assembled into fully functional pentamers as demonstrated by binding to GM1 ganglioside. Future work will involve testing of transgenic tomatoes for immunogenicity in mice following oral delivery.
Similar content being viewed by others
Introduction
Dengue is one the most common mosquitoes-borne human infectious diseases worldwide. It is caused by dengue virus belonging to the Flavivirus group, which includes viruses such as those causing yellow fever, West Nile, Zika and Japanese encephalitis. The dengue epidemic has increased rapidly since the 1950s and has emerged as a global problem with a high economic burden. One third of the world’s population lives in dengue-endemic areas, which span 100 countries within Asia, the Americas, the Western Pacific, Africa and Eastern Mediterranean regions. As many as 400 million people are infected annually, and the burden of dengue infection is likely higher than that reported by national surveillance systems due to unreported cases in developing countries. Dengue virus is a leading cause of illness and death, especially in children under 15 years of age (Gurugama et al. 2010; Sabchareon et al. 2012). To lower this risk substantially, an efficacious vaccine is urgently needed; however, a universal dengue vaccine is not yet available. There are four dengue virus serotypes, which have 60–75 % amino acid (aa) sequence homology. However, each of the four serotypes can cause dengue infection in an antigenically distinct manner, which can induce severe symptoms such as fatal dengue hemorrhagic fever (DHF) and dengue shock syndrome (DSS). Antibody-dependent enhancement (ADE) of infection can occur in vivo (Balsitis et al. 2010; Moi et al. 2013) due to increased viral uptake by cells mediated by non-neutralizing antibodies (Huang et al. 2016). Paradoxically, a protective immune response against one or two dengue virus serotypes could actually increase the risk of potentially fatal illness through ADE. Therefore, a successful dengue vaccine must be capable of simultaneously inducing high-level long-lasting immunity against all four serotypes.
Dengue virus consists of three structural proteins [capsid, membrane and envelope (E) proteins] and seven non-structural proteins (NS) (Guzman et al. 2010a). The E protein or domain III of E protein (EDIII, 299–393 aa) has been studied extensively as a subunit vaccine candidate (Guzman et al. 2010b; Ketloy et al. 2016; Wahala et al. 2012). NS proteins induce type-specific protective immunity and a majority of the CD4 and CD8 T cell responses (Halstead 2013). The recently developed tetravalent vaccine Dengvaxia, produced by Sanofi Pasteur, is based on live attenuated virus (McArthur and Edelman 2015; Sabchareon et al. 2012; Zust et al. 2013). Another live attenuated vaccine candidate, TV003, developed by the National Institute of Allergy and Infectious Diseases (NIAID) in the USA, was fully protective against dengue in a human challenge model (Kirkpatrick et al. 2016) and is currently undergoing a phase 3 clinical trial in Brazil. Other vaccine candidates based on DNA (Ketloy et al. 2016) and protein (Chiang et al. 2014) are also under consideration as viable alternatives to the attenuated virus-based vaccines.
Plant-based vaccines are an attractive possibility, owing to their superior safety profile and low production cost. Such vaccines have been explored for the past three decades, although none has yet been licensed. However, plant-based oral vaccines are of particular interest due to their potential for protecting the protein antigen in a gastric acid environment. The encapsulated antigens expressed in plant cells have been shown to be an efficient mode of antigen delivery to immune cells by oral administration in mice (Kwon et al. 2013; Limaye et al. 2006). In an experiment to induce tolerance, antibody formulated coagulation factor VIII, cholera toxin B (CTB)-heavy chain and CTB-C2 administered orally to gut-associated lymphoid tissue (GALT) demonstrated binding to dendritic cells in the lamina propria and Peyer’s patches of the small intestine (Su et al. 2010). A recent review by Daniell et al. listed many examples of plant-derived oral vaccines against human pathogens, including human papillomaviruses (HPVs), influenza virus, tuberculosis, hepatitis B virus (HBV), human immunodeficiency virus (HIV), rabies and malaria (Chan and Daniell 2015). Antigens from such human infectious agents have been introduced into plant cells subsequently tested as potential oral vaccines. In particular, tomatoes show great oral vaccine potential, as they contain tomato lectin, a known vehicle for gut antigen delivery, and alpha tomatine, a powerful plant endogenous adjuvant (Carreno-Gomez et al. 1999; Morrow et al. 2004). Therefore, we designed a tomato-derived dengue vaccine candidate and investigated its feasibility as a potential oral vaccine candidate.
Materials and methods
Transformation and regeneration of plants
Previously, we generated a DNA construct encoding cholera toxin B subunit fused to dengue virus E glycoprotein domain III (CTB–EDIII) with an endoplasmic reticulum (ER) retention signal peptide (Kim et al. 2010b, 2013). To develop a plant-based dengue vaccine, the plant expression vector containing the CTB–EDIII construct was transformed into tomatoes (Solanum lycopersicum) using the Agrobacterium-mediated gene transformation method. For tomato transformation, commercial tomato seeds were surface-sterilized using 70 % ethanol for 1 min and 10 % sodium hypochlorite with several drops of Tween 20 for 10 min, and then germinated on Murashige and Skoog (MS) basal medium supplemented with 3 % sucrose and 0.8 % plant agar (pH 5.7) at 25 °C in the dark for 5–6 days. The cotyledons were collected from the germinated plants under aseptic conditions, cut on both sides and incubated with Agrobacterium suspension solution for 15 min to allow penetration into the plant cells. To remove the remaining Agrobacterium from the surface, explants were washed twice with sterilized H2O and blotted with filter paper. The Agrobacterium-penetrated explants were co-cultured on MS solid medium in the dark for 2 days. The transformed explants were selected on MS medium containing antibiotics plus plant growth hormones (MS basic medium + 1 mg/L zeatin + 0.02 mg/L indole-3-acetic acid (IAA) + 100 mg/mL kanamycin + 300 mg/mL cefotaxime) and cultivated for 4–5 weeks. To obtain the regenerated plants with roots, the shoots induced from transformed explants were transferred to hormone-free MS solid medium. The plantlets with good developed roots and 7–10 cm in size were transferred to soil containing vermiculite and peatmoss and grown up in greenhouse (at 25 ± 2 °C under an intensity of 2000–3000 lx and with a photoperiod of 10 h).
PCR amplification of CTB–EDIII gene in transformed plants
Genomic DNA was isolated from wild-type and putative transgenic tomato leaf tissue using the DNeasy Plant Mini Kit (Qiagen, Valencia, CA, USA) to confirm integration of the target gene into the plant genome. The CTB–EDIII fusion gene was amplified using CTB–EDIII-specific primers (forward primer: 5′-GGT CTA GAG GAT CCG CCA CCA TGG TGA AG-3′ and reverse primer: 5′-GCG GTA CCT TTC TTG AAC CAG TTG AG-3′) by polymerase chain reaction (PCR) assay.
Northern blot analysis to detect CTB–EDIII transcripts
To confirm the expression of mRNA, total RNA was extracted from wild-type and transgenic tomato plants using TRIzol reagent (Molecular Research Center, Cincinnati, OH, USA) according to the manufacturer’s instructions. Total RNA samples (30 μg) were concentrated by ethanol precipitation, fractionated by formaldehyde–agarose gel electrophoresis and transferred onto Hybond N+ membrane (GE Healthcare Life Science Biotech, UK). The membrane was hybridized with a 32P-labeled CTB–EDIII probe using the Prime-a-Gene labeling system (Promega, Madison, WI, USA) at 65 °C in a hybridization incubator (FINEPCR Combi-H, Seoul, Korea). To visualize the transcripts of CTB–EDIII, the blotted membrane was exposed to X-ray film (Kodak, Rochester, NY, USA) after washing twice with 2× SSC (300 mM NaCl, 30 mM Na-citrate) buffer containing 0.1 and 1 % sodium dodecyl sulfate (SDS) for 15 min at 65 °C.
Immunoblot analysis of CTB–EDIII protein
To verify the expression of fusion protein in transgenic plants, total soluble protein (TSP) was extracted in protein extraction buffer (200 mM Tris–HCl, pH 8.0, 100 mM NaCl, 400 mM sucrose, 10 mM EDTA, 14 mM 2-mercaptoethanol, 1 mM phenylmethylsulfonyl fluoride and 0.05 % Tween 20). The extracted TSP was quantified by the Bradford protein assay (Bio-Rad, Hercules, CA, USA), and 50 µg was electrophoresed on 8 and 12 % polyacrylamide gels containing SDS, under non-reducing (NR) and reducing (R) conditions, respectively. The purified recombinant CTB or dengue EDIII proteins alone, and wild-type plant extract were used as controls for blotting. The separated protein bands were blotted onto Hybond C membranes (Promega) in transfer buffer (50 mM Tris, 40 mM glycine, and 20 % methanol) using a mini-transblot apparatus (Bio-Rad) at 130 mA for 2 h and detected with anti-CT antiserum (1:5000; Immunology Consultants Lab, Newberg, OR, USA) or anti-dengue monoclonal antibodies (1:2500; AbD Serotech, Oxford, UK). The proteins on the membrane were detected by alkaline phosphatase conjugated anti-rabbit IgG (S3731,1:5000; Promega) or anti-mouse (S372B, 1:5000; Promega) antibodies.
Detection of biological activity and quantification of CTB–EDIII fusion protein in transgenic plants
To detect the biological activity of CTB–EDIII produced in tomato plants, binding to monosialotetrahexosylganglioside (GM1) was detected using an enzyme-linked immunosorbent assay (ELISA). Nunc Maxisorp 96-well ELISA plates were coated with 3 µg/mL of GM1 (Sigma-Aldrich, St. Louis, MO, USA) in bicarbonate buffer (15 mM Na2CO3, 25 mM NaHCO3, pH 9.6) and incubated at 4 °C overnight. After blocking with 1 % BSA in PBS, triplicates samples including recombinant CTB as standard protein were added to the wells in twofold serial dilutions and incubated for 2 h at 37 °C. The plates were then washed and probed with the same antibodies used for immunoblot analysis. The ELISA plates were developed by adding alkaline phosphatase buffer [10 % (v/v) diethanol amine, 0.1 % MgCl2, 0.02 % sodium azide, pH 9.8] with one tablet of phosphate substrate (S0942-100TAB, Sigma-Aldrich), and the absorbance was read at 405 nm. The concentration of assembled CTB–EDIII was extrapolated from the standard curve obtained with known amounts of CTB–antibody complex. The analysis of variance was performed using the Excel statistical analysis program (Microsoft, Redmond, WA, USA).
Results
Transformation and regeneration of transgenic plants
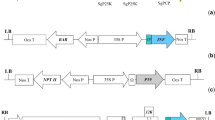
Tomato plants were transformed with Agrobacterium carrying the plant expression vector containing the CTB–EDIII gene construct. Kanamycin-resistant shoots formed at 4–5 weeks of growth on selection medium, and these shoots developed roots on MS hormone-free medium after 1 week. Figure 1a shows a typical transgenic shoot; root formation from a regenerated plant (in the bottle); maturation of plants transplanted to soil; and a transgenic tomato, respectively.
Regeneration of transgenic tomatoes and detection of the chimeric gene: cholera toxin B subunit fused to dengue virus E glycoprotein domain III (CTB–EDIII). a Regeneration of transgenic plants. The results show shoot induction on selection medium containing kanamycin; root formation from a regenerated plant (in the bottle); maturation of plants transplanted to soil; and growth of transgenic tomato. b Detection of CTB–EDIII fusion gene by genomic DNA polymerase chain reaction (PCR) in transformed plants. CTB–EDIII fused with the endoplasmic reticulum (ER) retention peptide (SEKDEL) was detectable at the expected size (734 bp) (indicated by an arrow beside the figure). Lane 1 plasmid DNA containing CTB–EDIII gene; lane 2 genomic DNA from wild-type plant; lanes 3–11 genomic DNA from transformed plants
Detection of CTB–EDIII fusion gene by genomic DNA PCR
Integration of the fusion genes into the nucleus of tomato cells was verified by genomic DNA PCR amplification using gene-specific primers. The 734 nucleotide fragment corresponding to CTB–EDIII was detected in transgenic but not wild-type plants (Fig. 1b). These result indicated that the CTB–EDIII gene was successfully inserted into the DNA of tomatoes following Agrobacterium-mediated transformation.
Detection of CTB–EDIII transcripts by northern blotting
The extracted RNA was analyzed for the presence of CTB–EDIII mRNA in transgenic plants. Positive signals for CTB–EDIII were detected in five of the seven plants analyzed, though there were variations in the intensity (Fig. 2a). The highest-expressing transgenic plant was selected for further protein analysis. Non-transformed wild-type plants showed no mRNA expression.
Detection of CTB–EDIII transcripts in transgenic plants. Northern blot analysis to detect CTB–EDIII transcripts. The expression of mRNA was confirmed at the expected size, indicated by an arrow (a). Total RNA separated on an agarose gel was stained by ethidium bromide (b). Lane 1 total RNA extract from wild-type plant; lanes 2–8 total RNA extracts from transgenic plants
Detection of CTB–EDIII fusion protein by immunoblotting
The synthesized and assembled fusion proteins were analyzed by Western blotting. The protein band at 130 kDa corresponding to the pentameric form was detected by both anti-CT and anti-dengue antibodies under NR conditions (Fig. 3, upper panels). These molecules were dissembled under R conditions giving rise to two protein bands at 26 and 30 kDa (Fig. 3, lower panels). An additional protein band at 60 kDa was detected by anti-CT antiserum under R conditions, likely corresponding to dimeric fusion protein. These results showed that CTB–EDIII protein introduced into tomato plants was successfully synthesized and formed molecular pentamers.
Detection of CTB–EDIII fusion protein in transgenic plants. Immunoblot analysis to detect the expression of CTB–EDIII under non-reducing (NR) or reducing (R) conditions. The results show a major protein band detected at 130 kDa under NR conditions and two monomeric forms at approximately 27 and 30 kDa under R conditions. Recombinant CTB protein (a) or dengue EDIII protein (b) purified from E. coli was used as positive control and wild-type plants as the negative control. The proteins were detected by anti-CT antiserum (a) and anti-dengue monoclonal antibodies (b). The expected band sizes are indicated by arrows. Molecular weight markers (in kDa) are shown on the left
Detection of biological activity and quantification of CTB–EDIII
The concentration and biological activity of the fusion protein was assessed in a GM1-binding ELISA assay. The binding to GM1 ganglioside was detected by both anti-CT and anti-dengue antibodies, though stronger signals were detected with anti-CT than anti-dengue antibodies (Fig. 4). In contrast, wild-type plants showed no binding. These results indicated that the CTB–EDIII fusion protein was properly assembled in transgenic tomato plants and was biological active, as demonstrated by binding to GM1 ganglioside, which is present on epithelial cells of the intestine. The expression level of the fusion proteins was found to be 0.015 % of the TSP (Fig. 5).
Determination of monosialotetrahexosylganglioside (GM1)-binding ability of CTB–EDIII produced in transgenic plants. GM1 enzyme-linked immunosorbent assay (ELISA) to detect a functional CTB–EDIII protein. The results show the CTB–EDIII bound to GM1-ganglioside. Protein extracts were diluted from 0.4 mg/mL of total soluble protein (TSP) for anti-CT antibody detection (a) or 0.8 mg/mL of TSP for anti-dengue antibody detection (b). Results are shown as the means of triplicate measurements ±SD
Quantification of CTB–EDIII fusion protein in transgenic plants. The level of biologically active CTB fusion proteins showing binding affinity to GM1. The amount of protein, calculated by comparing with known amounts of bacterial CTB–antibody complex, was found to be ~0.015 % of TSP in plants. Results are shown as the means of three measurements ±SD
Discussion
Dengue is an important viral infection producing a wide spectrum of illnesses ranging from mild dengue fever (DF) to potentially fatal symptoms such as DHF or DSS. Dengue is rapidly spreading worldwide from tropical and subtropical endemic regions owing to globalization, urbanization and climate change. This situation can be mitigated by proper treatment and a universal vaccine (Bennett et al. 2010; Messina et al. 2014). According to the World Health Organization (WHO) report in 2011, 100 million cases of DF and half a million cases of DHF occur annually, with an average case fatality rate of ~5 % (Gurugama et al. 2010). Dengvaxia®—a live, attenuated, tetravalent recombinant yellow fever-17D—dengue virus vaccine—has been licensed recently in Brazil, Mexico and the Philippines for use in individuals 9–45 years of age. The results of the vaccine efficacy trials performed in Asia and Latin America indicated 60.8 % overall efficacy against symptomatic dengue disease (Hadinegoro et al. 2015). A follow-up study indicated that there was an increased risk of hospitalization in individuals belonging to the under 9-year-old group (Hadinegoro et al. 2015). This increased risk is problematic because children are the most important age-group in terms of reducing the infection, as up to 90 % of patients with DHF are children less than 15 years of age (Gurugama et al. 2010). This outcome is most likely due to ADE as the vaccine formulation comprised four attenuated chimeric viruses from each serotype of dengue virus. Therefore, a global dengue vaccine for use in all age-groups is necessary for reducing the dengue mortality by 50 % and morbidity by 25 % by year 2020, as set out by the WHO (2009). Ideally, the vaccine should be efficient, inexpensive and safe, and should be easily applicable to young children. A plant-based mucosal (oral) vaccine could potentially meet these criteria.
Plants have often been used as delivery vehicles for testing mucosal vaccine candidates against human infections such as HBV, HIV, HPV, influenza virus, Mycobacterium tuberculosis and Plasmodium falciparum. In particular, edible plant vaccines delivered in the form of potato, lettuce and carrot have been explored for oral delivery by several research groups (Pniewski et al. 2011; Uvarova et al. 2013; Youm et al. 2010). Such vaccines were shown to be immunogenic in mice, and the antigens often formed particulate complexes such as virus-like particles (VLPs), which mimic authentic virions (Huang et al. 2005; Mason et al. 1996; Tacket et al. 2000). HPV antigen (HPVL1-E6/E7) expressed in tomatoes showed immunogenicity by inducing neutralizing antibodies and inhibiting tumor growth in mice (Monroy-Garcia et al. 2014). In this study, tomatoes were used not only to express dengue antigen fusion protein, but also to develop an oral vaccine against dengue infection.
Tomato is one of the most popular and valuable agricultural products in dengue-endemic areas, such as Africa and Asia. Tomato is particularly amenable for production of vaccines because it contains two important substances: alpha tomatine (an endogenous plant adjuvant) and tomato lectin (an immunogen by forming tomato lectin/microbial antigen complex) (Carreno-Gomez et al. 1999; Morrow et al. 2004). Alpha tomatine, a saponin contained in green tomatoes at high concentrations, can induce a strong immune response at low doses (Oda et al. 2000; Sun et al. 2007). Hwang et al. reported successful oral vaccination with hemagglutinin H5 antigen protein purified from transgenic plants plus 10 μg of adjuvant saponin. The vaccine induced protection against a highly pathogenic avian influenza virus in immunized mice (Lee et al. 2015). Thus, we hypothesize that transgenic tomato plants could similarly induce a strong immune response and protect against dengue infection by oral administration of green tomatoes containing CTB–EDIII fusion protein antigen.
Although oral immunization normally induces immunogenic tolerance, this can be overcome by employing various strategies including the use of exogenous adjuvants such as AB5 toxins and saponins, the application of genetic-encoded particles such as VLPs, or the use of receptor-targeting ligands (Kim et al. 2010a; Monroy-Garcia et al. 2014; Tochikubo et al. 1998). CTB, a proven adjuvant (Boyhan and Daniell 2011; Harokopakis et al. 1998; Mikschofsky et al. 2009), is also used as a ‘breaker’ of immunity in autoimmune diseases (Stratmann 2015). In a previous study, we demonstrated oral immunogenicity against a fusion protein comprised of CTB and porphyromonas gingivalis fimbrial protein antigen (expressed in E. coli) (Kim et al. 2009), and evaluated its oligomerization and biological activity when expressed in plants (Kim et al. 2010b, 2013). We found that the orally administrated CTB fusion proteins could be internalized by M cells and enterocytes in Peyer’s patches (Kim et al. 2013).
In the present study, CTB was genetically fused to dengue antigen and directed to the ER for expression. The fusion protein was expressed in tomato plants, and we hypothesized that consuming the tomato would enhance the oral immune response by increasing the chance of interaction with GM1 ganglioside on epithelial cells of the gut. The assembled CTB–EDIII fusion protein was verified by immunoblotting and GM1 ELISA assays. The 130-kDa expression product corresponding to the pentameric form was detected under NR conditions. Two major protein bands were observed under R conditions (Fig. 3), likely corresponding to non-glycosylated and glycosylated monomers (as there are two N-glycosylation sites in the CTB sequence). The CTB–EDIII protein in the ER was largely intact, and evidence of slight degradation was observed in reduced samples by anti-CT antiserum (Fig. 3a) but not by anti-dengue antibodies (Fig. 3b). These results suggested that the CTB–EDIII fusion protein could be stably expressed and assembled efficiently into pentamers in the ER. The CTB–EDIII protein showed a strong affinity for GM1-ganglioside, which is an important characteristic for a potential oral immunogen. Although the expression level of biologically active CTB–EDIII was relatively low, at 0.015 % of TSP in fresh plants, we anticipate that much higher yields could be achieved in freeze-dried green tomato fruits. Therefore, we expect that this transgenic tomato-derived CTB–EDIII could induce a strong immune response against dengue antigen by oral vaccination.
This study showed that CTB–EDIII could be expressed and assembled into a biologically active form in transgenic tomato plants. These transgenic tomatoes will be evaluated for their immunogenic potential in mice as an oral vaccine candidate against dengue infection.
References
Balsitis SJ, Williams KL, Lachica R, Flores D, Kyle JL, Mehlhop E, et al. (2010) Lethal Antibody Enhancement of Dengue Disease in Mice Is Prevented by Fc Modification. PLoS Pathog 6(2): e1000790. doi:10.1371/journal.ppat.1000790
Bennett SN, Drummond AJ, Kapan DD, Suchard MA, Munoz-Jordan JL, Pybus OG, Holmes EC, Gubler DJ (2010) Epidemic dynamics revealed in dengue evolution. Mol Biol Evol 27:811–818
Boyhan D, Daniell H (2011) Low-cost production of proinsulin in tobacco and lettuce chloroplasts for injectable or oral delivery of functional insulin and C-peptide. Plant Biotechnol J 9:585–598
Carreno-Gomez B, Woodley JF, Florence AT (1999) Studies on the uptake of tomato lectin nanoparticles in everted gut sacs. Int J Pharm 183:7–11
Chan HT, Daniell H (2015) Plant-made oral vaccines against human infectious diseases—are we there yet? Plant Biotechnol J 13:1056–1070
Chiang CY, Hsieh CH, Chen MY, Tsai JP, Liu HH, Liu SJ, Chong P, Leng CH, Chen HW (2014) Recombinant lipidated dengue-4 envelope protein domain III elicits protective immunity. Vaccine 32:1346–1353
Gurugama P, Garg P, Perera J, Wijewickrama A, Seneviratne SL (2010) Dengue viral infections. Indian J Dermatol 55:68–78
Guzman MG, Halstead SB, Artsob H, Buchy P, Jeremy F, Gubler DJ, Hunsperger E, Kroeger A, Margolis HS, Martinez E, Nathan MB, Pelegrino JL, Cameron S, Yoksan S, Peeling RW (2010a) Dengue: a continuing global threat. Nat Rev Microbiol S7–S16. doi:10.1038/nrmicro2460
Guzman MG, Hermida L, Bernardo L, Ramirez R, Guillen G (2010b) Domain III of the envelope protein as a dengue vaccine target. Expert Rev Vaccines 9:137–147
Hadinegoro SR, Arredondo-García JL, Capeding MR, Deseda C, Chotpitayasunondh T, Dietze R, Hj Muhammad Ismail HI, Reynales H, Limkittikul K, Rivera-Medina DM, Tran HN, Bouckenooghe A, Chansinghakul D, Cortés M, Fanouillere K, Forrat R, Frago C, Gailhardou S, Jackson N, Noriega F, Plennevaux E, Wartel TA, Zambrano B, Saville M (2015) Efficacy and Long-Term Safety of a Dengue Vaccine in Regions of Endemic Disease. New Engl J Medicine 373:1195–1206
Halstead SB (2013) Identifying protective dengue vaccines: guide to mastering an empirical process. Vaccine 31:4501–4507
Harokopakis E, Hajishengallis G, Michalek SM (1998) Effectiveness of liposomes possessing surface-linked recombinant B subunit of cholera toxin as an oral antigen delivery system. Infect Immun 66:4299–4304
Huang Z, Elkin G, Maloney BJ, Beuhner N, Arntzen CJ, Thanavala Y, Mason HS (2005) Virus-like particle expression and assembly in plants: hepatitis B and Norwalk viruses. Vaccine 23:1851–1858
Huang X, Yue Y, Li D, Zhao Y, Qiu L, Chen J, Pan Y, Xi J, Wang X, Sun Q, Li Q (2016) Antibody-dependent enhancement of dengue virus infection inhibits RLR-mediated Type-I IFN-independent signalling through upregulation of cellular autophagy. Sci Rep 6:22303
Ketloy C, Keelapang P, Prompetchara E, Suphatrakul A, Puttikhunt C, Kasinrerk W, Konishi E, Sittisombut N, Ruxrungtham K (2016) Strategies to improve the immunogenicity of prM+E dengue virus type-2 DNA vaccine. Asian Pac J Allergy Immunol. doi:10.12932/AP0728
Kim TG, Huy NX, Kim MY, Jeong DK, Jang YS, Yang MS, Langridge WHR, Lee JY (2009) Immunogenicity of a cholera toxin B subunit Porphyromonas gingivalis fimbrial antigen fusion protein expressed in E. coli. Mol Biotechnol 41:157–164
Kim SH, Seo KW, Kim J, Lee KY, Jang YS (2010a) The M cell-targeting ligand promotes antigen delivery and induces antigen-specific immune responses in mucosal vaccination. J Immunol 185:5787–5795
Kim TG, Kim MY, Yang MS (2010b) Cholera toxin B subunit-domain III of dengue virus envelope glycoprotein E fusion protein production in transgenic plants. Protein Expr Purif 74:236–241
Kim MY, Chung ND, Yang MS, Kim TG (2013) Expression of a cholera toxin B subunit and consensus dengue virus envelope protein domain III fusion gene in transgenic rice callus. Plant Cell Tissue Org 112:311–320
Kirkpatrick BD, Whitehead SS, Pierce KK, Tibery CM, Grier PL, Hynes NA, Larsson CJ, Sabundayo BP, Talaat KR, Janiak A, Carmolli MP, Luke CJ, Diehl SA, Durbin AP (2016) The live attenuated dengue vaccine TV003 elicits complete protection against dengue in a human challenge model. Sci Transl Med 8(330). doi: 10.1126/scitranslmed.aaf1517
Kwon KC, Verma D, Singh ND, Herzog R, Daniell H (2013) Oral delivery of human biopharmaceuticals, autoantigens and vaccine antigens bioencapsulated in plant cells. Adv Drug Deliv Rev 65:782–799
Lee G, Na YJ, Yang BG, Choi JP, Seo YB, Hong CP, Yun CH, Kim DH, Sohn EJ, Kim JH, Sung YC, Kim YK, Jang MH, Hwang I (2015) Oral immunization of haemaggulutinin H5 expressed in plant endoplasmic reticulum with adjuvant saponin protects mice against highly pathogenic avian influenza A virus infection. Plant Biotechnol J 13:62–72
Limaye A, Koya V, Samsam M, Daniell H (2006) Receptor-mediated oral delivery of a bioencapsulated green fluorescent protein expressed in transgenic chloroplasts into the mouse circulatory system. Faseb J 20:959–961
Mason HS, Ball JM, Shi JJ, Jiang X, Estes MK, Arntzen CJ (1996) Expression of Norwalk virus capsid protein in transgenic tobacco and potato and its oral immunogenicity in mice. Proc Natl Acad Sci USA 93:5335–5340
McArthur MA, Edelman R (2015) A promising, single-dose, live attenuated tetravalent dengue vaccine candidate. J Infect Dis 212:681–683
Messina JP, Brady OJ, Scott TW, Zou C, Pigott DM, Duda KA, Bhatt S, Katzelnick L, Howes RE, Battle KE, Simmons CP, Hay SI (2014) Global spread of dengue virus types: mapping the 70 year history. Trends Microbiol 22:138–146
Mikschofsky H, König P, Keil GM, Hammer M, Schirrmeier H, Broer I (2009) Cholera toxin B (CTB) is functional as an adjuvant for cytoplasmatic proteins if directed to the endoplasmatic reticulum (ER), but not to the cytoplasm of plants. Plant Sci 177:35–42
Moi ML, Takasaki T, Saijo M, Kurane I (2013) Dengue virus infection-enhancing activity of undiluted sera obtained from patients with secondary dengue virus infection. Trans R Soc Trop Med Hyg 107:51–58
Monroy-Garcia A, Gomez-Lim MA, Weiss-Steider B, Hernandez-Montes J, Huerta-Yepez S, Rangel-Santiago JF, Santiago-Osorio E, Garcia MDM (2014) Immunization with an HPV-16 L1-based chimeric virus-like particle containing HPV-16 E6 and E7 epitopes elicits long-lasting prophylactic and therapeutic efficacy in an HPV-16 tumor mice model. Arch Virol 159:291–305
Morrow WJW, Yang YW, Sheikh NA (2004) Immunobiology of the Tomatine adjuvant. Vaccine 22:2380–2384
Oda K, Matsuda H, Murakami T, Katayama S, Ohgitani T, Yoshikawa M (2000) Adjuvant and haemolytic activities of 47 saponins derived from medicinal and food plants. Biol Chem 381:67–74
Pniewski T, Kapusta J, Bociag P, Wojciechowicz J, Kostrzak A, Gdula M, Fedorowicz-Stronska O, Wojcik P, Otta H, Samardakiewicz S, Wolko B, Plucienniczak A (2011) Low-dose oral immunization with lyophilized tissue of herbicide-resistant lettuce expressing hepatitis B surface antigen for prototype plant-derived vaccine tablet formulation. J Appl Genet 52:125–136
Sabchareon A, Sirivichayakul C, Limkittikul K, Chanthavanich P, Suvannadabba S, Jiwariyavej V, et al. (2012) Dengue Infection in Children in Ratchaburi, Thailand: A Cohort Study. I. Epidemiology of Symptomatic Acute Dengue Infection in Children, 2006–2009. PLoS Negl Trop Dis 6(7):e1732. doi:10.1371/journal.pntd.0001732
Sabchareon A, Wallace D, Sirivichayakul C, Limkittikul K, Chanthavanich P, Suvannadabba S, Jiwariyavej V, Dulyachai W, Pengsaa K, Wartel TA, Moureau A, Saville M, Bouckenooghe A, Viviani S, Tornieporth NG, Lang J (2012) Protective efficacy of the recombinant, live-attenuated, CYD tetravalent dengue vaccine in Thai schoolchildren: a randomised, controlled phase 2b trial. Lancet 380:1559–1567
Stratmann T (2015) Cholera toxin subunit B as adjuvant—an accelerator in protective immunity and a break in autoimmunity. Vaccines 3:579–596
Su RJ, Epp A, Latchman Y, Bolgiano D, Pipe SW, Josephson NC (2010) Suppression of FVIII inhibitor formation in hemophilic mice by delivery of transgene modified apoptotic fibroblasts. Mol Ther 18:214–222
Sun JH, Hu S, Song XM (2007) Adjuvant effects of protopanaxadiol and protopanaxatriol saponins from ginseng roots on the immune responses to ovalbumin in mice. Vaccine 25:1114–1120
Tacket CO, Mason HS, Losonsky G, Estes MK, Levine MM, Arntzen CJ (2000) Human immune responses to a novel Norwalk virus vaccine delivered in transgenic potatoes. J Infect Dis 182:302–305
Tochikubo K, Isaka M, Yasuda Y, Kozuka S, Matano K, Miura Y, Taniguchi T (1998) Recombinant cholera toxin B subunit acts as an adjuvant for the mucosal and systemic responses of mice to mucosally co-administered bovine serum albumin. Vaccine 16:150–155
Uvarova EA, Belavin PA, Permyakova NV, Zagorskaya AA, Nosareva OV, Kakimzhanova AA, Deineko EV (2013) Oral immunogenicity of plant-made Mycobacterium tuberculosis ESAT6 and CFP10. Biomed Res Int 316304. http://doi.org/10.1155/2013/316304
Wahala WM, Huang C, Butrapet S, White LJ, de Silva AM (2012) Recombinant dengue type 2 viruses with altered e protein domain III epitopes are efficiently neutralized by human immune sera. J Virol 86:4019–4023
World Health Organization and the Special Programme for Research and Training in Tropical Diseases (TDR) (2009) Dengue: guidelines for diagnosis, treatment, prevention and control. WHO Press, pp 1–147
Youm JW, Won YS, Jeon JH, Moon KB, Kim HC, Shin KS, Joung H, Kim HS (2010) Antibody responses in mice stimulated by various doses of the potato-derived major surface antigen of hepatitis B virus. Clin Vaccine Immunol 17:2029–2032
Zust R, Dong H, Li XF, Chang DC, Zhang B, Balakrishnan T, Toh YX, Jiang T, Li SH, Deng YQ, Ellis BR, Ellis EM, Poidinger M, Zolezzi F, Qin CF, Shi PY, Fink K (2013) Rational design of a live attenuated dengue vaccine: 2′-o-methyltransferase mutants are highly attenuated and immunogenic in mice and macaques. PLoS Pathog 9:e1003521
Acknowledgments
This study was supported by NRF-2014K1B1A1073861 through the National Research Foundation (NRF) funded by the Korean Ministry of Science, ICT and Future Planning and by the Advanced Production Technology Development Program (312037-05), Ministry of Agriculture, Food and Rural Affairs, Republic of Korea.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, MY., Kim, BY. & Yang, MS. Synthesis and assembly of dengue virus envelope protein fused to cholera toxin B subunit into biologically active oligomers in transgenic tomato (Solanum lycopersicum). Plant Biotechnol Rep 10, 219–226 (2016). https://doi.org/10.1007/s11816-016-0398-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11816-016-0398-3