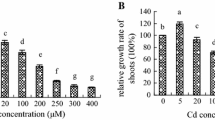
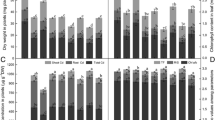
Abstract
Nitric oxide (NO), a multifunctional gaseous molecule, mediates a variety of responses to biotic and abiotic stresses. The effects of exogenous NO on rice (Oryza sativa cv. ‘Zhonghua 11’) growth under mercuric chloride (HgCl2) stress were investigated. The results showed that 60 μM Hg significantly inhibited the root elongation of rice plantlets after seed germination. While 100 μM or 200 μM sodium nitroprusside (SNP, a donor of NO) could increase the root length by attenuating the effects of 2,3,5-triiodobenzoic acid (TIBA) and Hg, which indicated the role of NO in auxin transport-promoting in roots. On the other hand, SNP decreased the absorption and transportation of Hg in roots and shoots of rice seedlings at five-leaf stage. Moreover, the levels of superoxide radical (O2·−) and hydrogen peroxide (H2O2) in leaves were also decreased significantly. However, the activities of antioxidant enzymes were not enhanced by SNP. Moreover, NO promoted the growth of rice plantlets under Hg stress even when superoxide dismutase (SOD, EC 1.15.1.1) or catalase (CAT, 1.11.1.6) activity was inhibited by diethyldithiocarbamate (DDC, an inhibitor of SOD) or 3-amino-1,2,4-triazole (AT, an inhibitor of catalase), respectively. These results confirmed that NO could act as the direct quencher of O2·− and then prevent the oxidative damage caused by Hg ion in leaves.






Similar content being viewed by others
Abbreviations
- Hg:
-
Mercury
- NO:
-
Nitric oxide
- SNP:
-
Sodium nitroprusside
- ROS:
-
Reactive oxygen species
- O2·− :
-
Superoxide radical
- H2O2 :
-
Hydrogen peroxide
- SOD:
-
Superoxide dismutase
- CAT:
-
Catalase
- DDC:
-
Diethyldithiocarbamate
- AT:
-
3-amino-1,2,4-triazole
- TIBA:
-
2,3,5-triiodobenzoic acid
References
Abat JK, Deswal R (2013) Nitric oxide modulates the expression of proteins and promotes epiphyllous bud differentiation in Kalanchoe pinnata. J Plant Growth Regul 32:92–101
Aebi HE (1983) Catalase. In: Bergmeyer HU (ed) Methods of enzymatic analysis. Verlag Chemie, Weinheim, pp 273–285
Arasimowicz-Jelonek M, Floryszak-Wieczorek J, Gwóźdź EA (2011) The message of nitric oxide in cadmium challenged plants. Plant Sci 181:612–620
Beauchamp C, Fridovich I (1971) Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem 44:276–287
Besson-Bard A, Pugin A, Wendehenne D (2008) New insights into nitric oxide signaling in plants. Annu Rev Plant Biol 59:21–39
Cargnelutti D, Tabaldi LA, Spanevello RM, de Oliveira Jucoski G, Battisti V, Redin M, Redin M, Linares CEB, Dressler VL, de Moraes Flores ÉM, Nicoloso FT, Morsch VM, Schetinger MRC (2006) Mercury toxicity induces oxidative stress in growing cucumber seedlings. Chemosphere 65:999–1006
Chen J, Yang ZM (2012) Mercury toxicity, molecular response and tolerance in higher plants. Biometals 25:847–857
Chen Z, Pan YH, Wang SS, Ding YF, Yang WJ, Zhu C (2012) Overexpression of a protein disulfide isomerase-like protein from Methanothermobacter thermoautotrophicum enhances mercury tolerance in transgenic rice. Plant Sci 197:10–20
Cho U, Park J (2000) Mercury-induced oxidative stress in tomato seedlings. Plant Sci 156:1–9
Fernández-Marcos M, Sanz L, Lorenzo O (2012) Nitric oxide: an emerging regulator of cell elongation during primary root growth. Plant Signal Behav 7:196–200
Gao S, Ou-yang C, Tang L, Zhu JQ, Xu Y, Wang SH, Chen F (2010) Growth and antioxidant responses in Jatropha curcas seedling exposed to mercury toxicity. J Hazard Mater 182:591–597
Guo B, Liang YC, Zhu YG, Zhao FJ (2007) Role of salicylic acid in alleviating oxidative damage in rice roots (Oryza sativa) subjected to cadmium stress. Environ Pollut 147:743–749
Guo K, Xia K, Yang ZM (2008) Regulation of tomato lateral root development by carbon monoxide and involvement in auxin and nitric oxide. J Exp Bot 59:3443–3452
Han YH, Moon HJ, You BR, Kim SZ, Kim SH, Park WH (2009) The effects of buthionine sulfoximine, diethyldithiocarbamate or 3-amino-1,2,4-triazole on propyl gallate-treated HeLa cells in relation to cell growth, reactive oxygen species and glutathione. Int J Mol Med 24:261–268
He HY, He LF, Gu MH, Li XF (2012) Nitric oxide improves aluminum tolerance by regulating hormonal equilibrium in the root apices of rye and wheat. Plant Sci 183:123–130
Inukai Y, Sakamoto T, Ueguchi-Tanaka M, Shibata Y, Gomi K, Umemura I, Hasegawa Y, Ashikari M, Kitano H, Matsuoka M (2005) Crown rootless1, which is essential for crown root formation in rice, is a target of AUXIN RESPONSE FACTOR in auxin signaling. Plant Cell 17:1387–1396
Jana SCM (1981) Glycolate metabolism of three submerged aquatic angiosperms during aging. Aquat Bot 12:345–354
Kazemi N, Khavari-Nejad RA, Fahimi H, Saadatmand S, Nejad-Sattari T (2010) Effects of exogenous salicylic acid and nitric oxide on lipid peroxidation and antioxidant enzyme activities in leaves of Brassica napus L. under nickel stress. Sci Hortic 126:402–407
Kim HS, Jung MC (2012) Mercury contamination in agricultural soils from abandoned metal mines classified by geology and mineralization. Environ Geochem Health 34(Suppl 1):55–69
Kopyra M, Gwóźdź EA (2003) Nitric oxide stimulates seed germination and counteracts the inhibitory effect of heavy metals and salinity on root growth of Lupinus luteus. Plant Physiol Biochem 41:1011–1017
Lanteri ML, Pagnussat GC, Lamattina L (2006) Calcium and calcium-dependent protein kinases are involved in nitric oxide- and auxin-induced adventitious root formation in cucumber. J Exp Bot 57:1341–1351
Lushchak V, Semchyshyn H, Lushchak O, Mandryk S (2005) Diethyldithiocarbamate inhibits in vivo Cu, Zn-superoxide dismutase and perturbs free radical processes in the yeast Saccharomyces cerevisiae cells. Biochem Biophys Res Commun 338:1739–1744
Meng DK, Chen J, Yang ZM (2011) Enhancement of tolerance of Indian mustard (Brassica juncea) to mercury by carbon monoxide. J Hazard Mater 186:1823–1929
Mishra SKSD, Singhal GS (1991) Interrelationship between salt and light stress on the primary process of photosynthesis. J Plant Physiol 138:92–96
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880
Pagnussat GC, Simontacchi M, Puntarulo S, Lamattina L (2002) Nitric oxide is required for root organogenesis. Plant Physiol 129:954–956
Pagnussat GC, Lanteri ML, Lamattina L (2003) Nitric oxide and cyclic GMP are messengers in the indole acetic acid-induced adventitious rooting process. Plant Physiol 132:1241–1248
Rao MV, Paliyath G, Ormrod DP, Murr DP, Watkins CB (1997) Influence of salicylic acid on H2O2 production, oxidative stress, and H2O2-metabolizing enzymes. Salicylic acid-mediated oxidative damage requires H2O2. Plant Physiol 115:137–149
Sahu GKUS, Sahoo BB (2012) Mercury induced phytotoxicity and oxidative stress in wheat (Triticum aestivum L.) plants. Physiol Mol Biol Plants 18:21–31
Saxena I, Shekhawat GS (2013) Nitric oxide (NO) in alleviation of heavy metal induced phytotoxicity and its role in protein nitration. Nitric Oxide 32:13–20
Shiyab S, Chen J, Han FXX, Monts DL, Matta FB, Gu MM, Su Y, Masad MA (2009) Mercury-induced oxidative stress in Indian mustard (Brassica juncea L.). Environ Toxicol 24:462–471
Singh HP, Batish DR, Kaur G, Arora K, Kohli RK (2008) Nitric oxide (as sodium nitroprusside) supplementation ameliorates Cd toxicity in hydroponically grown wheat roots. Environ Exp Bot 63:158–167
Singh HP, Kaur S, Batish DR, Sharma VP, Sharma N, Kohli RK (2009) Nitric oxide alleviates arsenic toxicity by reducing oxidative damage in the roots of Oryza sativa (rice). Nitric Oxide 20:289–297
Sunderland EM, Selin NE (2013) Future trends in environmental mercury concentrations: implications for prevention strategies. Environ Health 12:2–5
Wang YS, Yang ZM (2005) Nitric oxide reduces aluminum toxicity by preventing oxidative stress in the roots of Cassia tora L. Plant Cell Physiol 46:1915–1923
Xiong J, An L, Lu H, Zhu C (2009a) Exogenous nitric oxide enhances cadmium tolerance of rice by increasing pectin and hemicellulose contents in root cell wall. Planta 230:755–765
Xiong J, Lu H, Lu KX, Duan YX, An LY, Zhu C (2009b) Cadmium decreases crown root number by decreasing endogenous nitric oxide, which is indispensable for crown root primordia initiation in rice seedlings. Planta 230:599–610
Yadav S, David A, Baluška F, Bhatla SC (2013) Rapid auxin-induced nitric oxide accumulation and subsequent tyrosine nitration of proteins during adventitious root formation in sunflower hypocotyls. Plant Signal Behav 8:e23196
Yoshida S, Forno DA, Cock JH, Gomez KA (1976) Laboratory manual for physiological studies of rice. International Rice Research Institute, Los Banõs
Zhang LP, Mehta SK, Liu ZP, Yang ZM (2008) Copper-induced proline synthesis is associated with nitric oxide generation in Chlamydomonas reinhardtii. Plant Cell Physiol 49:411–419
Zhang L, Chen Z, Zhu C (2012) Endogenous nitric oxide mediates alleviation of cadmium toxicity induced by calcium in rice seedlings. J Environ Sci 24:940–948
Zhou ZS, Guo K, Elbaz AA, Yang ZM (2009) Salicylic acid alleviates mercury toxicity by preventing oxidative stress in roots of Medicago sativa. Environ Exp Bot 65:27–34
Acknowledgments
This research was supported by the Zhejiang Provincial Natural Science Foundation of China (Grant NO: 3100246) and Taizhou University’s Scientific Research Project (2014PY024).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest and all authors have read and approved the final manuscript.
Additional information
Communicated by S. Srivastava.
Rights and permissions
About this article
Cite this article
Chen, Z., Zhang, L. & Zhu, C. Exogenous nitric oxide mediates alleviation of mercury toxicity by promoting auxin transport in roots or preventing oxidative stress in leaves of rice seedlings. Acta Physiol Plant 37, 194 (2015). https://doi.org/10.1007/s11738-015-1931-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-015-1931-7