Abstract
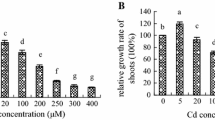
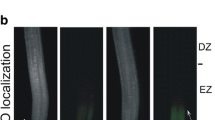
Cadmium (Cd) is toxic to crown roots (CR), which are essential for maintaining normal growth and development in rice seedlings. Nitric oxide (NO) is an important signaling molecule that plays a pivotal role in plant root organogenesis. Here, the effects of Cd on endogenous NO content and root growth conditions were studied in rice seedlings. Results showed that similar to the NO scavenger, cPTIO, Cd significantly decreased endogenous NO content and CR number in rice seedlings, and these decreases were recoverable with the application of sodium nitroprusside (SNP, a NO donor). Microscopic analysis of root collars revealed that treatment with Cd and cPTIO inhibited CR primordia initiation. In contrast, although SNP partially recovered Cd-caused inhibition of CR elongation, treatment with cPTIO had no effect on CR elongation. l-NMMA, a widely used nitric oxide synthase (NOS) inhibitor, decreased endogenous NO content and CR number significantly, while tungstate, a nitrate reductase (NR) inhibitor, had no effect on endogenous NO content and CR number. Moreover, enzyme activity assays indicated that treatment with SNP inhibited NOS activity significantly, but had no effect on NR activity. All these results support the conclusions that a critical endogenous NO concentration is indispensable for rice CR primordia initiation rather than elongation, NOS is the main source for endogenous NO generation, and Cd decreases CR number by inhibiting NOS activity and thus decreasing endogenous NO content in rice seedlings.







Similar content being viewed by others
Abbreviations
- AR:
-
Adventitious roots
- CR:
-
Crown roots
- DTT:
-
Dithiothreitol
- EDTA:
-
Ethylenediaminetetraacetic acid
- FAD:
-
Flavin adenine dinucleotide
- FMN:
-
Flavin mononucleotide
- LR:
-
Lateral roots
- NO:
-
Nitric oxide
- NOS:
-
Nitric oxide synthase
- NR:
-
Nitrate reductase
- PR:
-
Primary root
- SNP:
-
Sodium nitroprusside
References
Barroso JB, Corpas FJ, Carreras A, Rodríguez-Serrano M, Esteban FJ, Fernández-Ocaña A, Chaki M, Romero-Puertas MC, Valderrama R, Sandalio LM, del Río LA (2006) Localization of S-nitrosoglutathione and expression of S-nitrosoglutathione reductase in pea plants under cadmium stress. J Exp Bot 57:1785–1793
Bartha B, Kolbert Z, Erdei L (2005) Nitric oxide production induced by heavy metals in Brassica juncea L. Czern. and Pisum sativum L. Acta Biol Szeged 49:9–12
Beligni MV, Lamattina L (2002) Nitric oxide interferes with plant photo-oxidative stress by detoxifying reactive oxygen species. Plant Cell Environ 25:737–748
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem 72:248–254
Chaki M, Fernández-Ocaña AM, Valderrama R, Carreras A, Esteban FJ, Luque F, Gómez-Rodríguez MV, Begara-Morales JC, Corpas FJ, Barroso JB (2009) Involvement of reactive nitrogen and oxygen species (RNS and ROS) in sunflower–mildew interaction. Plant Cell Physiol 50:265–279
Corpas FJ, Barroso JB, Carreras A, Valderrama R, Palma JM, León AM, Sandalio LM, del Río LA (2006) Constitutive arginine-dependent nitric oxide synthase activity in different organs of pea seedlings during plant development. Planta 224:246–254
Correa-Aragunde N, Graziano M, Lamattina L (2004) Nitric oxide plays a central role in determining lateral root development in tomato. Planta 218:900–905
Foresi NP, Laxalt AM, Tonón CV, Casalongué CA, Lamattina L (2007) Extracellular ATP induces nitric oxide production in tomato cell suspensions. Plant Physiol 145:589–592
Fusconi A, Repetto O, Bona E, Massa N, Gallo C, Dumas-Gaudot E, Berta G (2006) Effects of cadmium on meristem activity and nucleus ploidy in roots of Pisum sativum L. cv. Frisson seedlings. Environ Exp Bot 58:253–260
Fusconi A, Gallo C, Camusso W (2007) Effects of cadmium on root apical meristems of Pisum sativum L.: cell viability, cell proliferation and microtubule pattern as suitable markers for assessment of stress pollution. Mutat Res Genet Toxicol Environ Mutagen 632:9–19
Furchgott RF (1995) Special topics: nitric oxide. Annu Rev Physiol 57:659–682
Gouvêa CMCP, Souza JF, Magalhães ACN, Martins IS (1997) NO-releasing substances that induce growth elongation in maize root segments. Plant Growth Regul 21:183–187
Groppa MD, Rosales EP, Iannone MF, Benavides MP (2008) Nitric oxide, polyamines and Cd-induced phytotoxicity in wheat roots. Phytochemistry 69:2609–2615
Guo B, Liang YC, Zhu YG, Zhao FJ (2007) Role of salicylic acid in alleviating oxidative damage in rice roots (Oryza sativa) subjected to cadmium stress. Environ Pollut 147:743–749
Hochholdinger F, Park WJ, Sauer M, Woll K (2004) From weeds to crops: genetic analysis of root development in cereals. Trends Plant Sci 9:42–48
Hsu YT, Kao CH (2005) Abscisic acid accumulation and cadmium tolerance in rice seedlings. Physiol Plant 124:71–80
Inukai Y, Miwa M, Nagato Y, Kitano H, Yamauchi A (2001) Characterization of rice mutants deficient in the formation of crown roots. Breed Sci 51:123–129
Inukai Y, Sakamoto T, Ueguchi-Tanaka M, Shibata Y, Gomi K, Umemura I, Hasegawa Y, Ashikari M, Kitano H, Matsuoka M (2005) Crown rootless1, which is essential for crown root formation in rice, is a target of AUXIN RESPONSE FACTOR in auxin signaling. Plant Cell 17:1387–1396
Itoh J, Nonomura K, Ikeda K, Yamaki S, Inukai Y, Yamagishi H, Kitano H, Nagato Y (2005) Rice plant development: from zygote to spikelet. Plant Cell Physiol 46:23–47
Kaufman PB (1959) Development of the shoot of Oryza sativa L. III. Early stages in histogenesis of the stem and ontogeny of the adventitious root. Phytomorphology 9:382–404
Kawata S, Harada J (1975) On the development of the crown root primordial in rice plants. Proc Crop Sci Soc Jpn 45:438–457
Kim DY, Bovet L, Maeshima M, Martinoia E, Lee Y (2007) The ABC transporter AtPDR8 is a cadmium extrusion pump conferring heavy metal resistance. Plant J 50:207–218
Kopyra M, Gwóźdź E (2003) Nitric oxide stimulates seed germination and counteracts the inhibitory effect of heavy metals and salinity on root growth of Lupinus luteus. Plant Physiol Biochem 441:1011–1017
Laspina NV, Groppa MD, Tomaro ML, Benavides MP (2005) Nitric oxide protects sunflower leaves against Cd-induced oxidative stress. Plant Sci 169:323–330
Liu HJ, Wang SF, Yu XB, Yu J, He XW, Zhang SL, Shou HX, Wu P (2005) ARL1, a LOB-domain protein required for adventitious root formation in rice. Plant J 43:47–56
Lombardo MC, Graziano M, Polacco JC, Lamattina L (2006) Nitric oxide functions as a positive regulator of root hair development. Plant Signal Behav 1:28–33
Lozano-Rodríguez E, Hernández LE, Bonay P, Carpena-Ruiz RO (1997) Distribution of cadmium in shoot and root tissues of maize and pea plants: physiological disturbances. J Exp Bot 48:123–128
Malamy JE, Benfey PN (1997) Down and out in Arabidopsis: the formation of lateral roots. Trends Plant Sci 2:390–396
Metwally A, Finkemeier I, Georgi M, Dietz K (2003) Salicylic acid alleviates the cadmium toxicity in barley seedlings. Plant Physiol 132:272–281
Neill SJ, Desikan R, Hancock JT (2003) Nitric oxide signalling in plants. New Phytol 159:11–35
Neill S, Bright J, Desikan R, Hancock J, Harrison J, Wilson I (2008) Nitric oxide evolution and perception. J Exp Bot 59:25–35
Pagnussat GC, Simontacchi M, Puntarulo S, Lamattina L (2002) Nitric oxide is required for root organogenesis. Plant Physiol 129:954–956
Pagnussat GC, Lanteri ML, Lamattina L (2003) Nitric oxide and cyclic GMP are messengers in the indole acetic acid-induced adventitious rooting process. Plant Physiol 132:1241–1248
Pagnussat GC, Lanteri ML, Lombardo MC, Lamattina L (2004) Nitric oxide mediates the indole acetic acid induction activation of a mitogen-activated protein kinase cascade involved in adventitious root development. Plant Physiol 135:279–285
Perrine-Walker FM, Gartner E, Hocart CH, Becker A, Rolfe BG (2007) Rhizobium-initiated rice growth inhibition caused by nitric oxide accumulation. Mol Plant Microbe Interact 20:283–292
Rodríguez-Serrano M, Romero-Puertas MC, Zabalza A, Corpas FJ, Gómez M, del Río LA, Sandalio LM (2006) Cadmium effect on oxidative metabolism of pea (Pisum sativum L.) roots. Imaging of reactive oxygen species and nitric oxide accumulation in vivo. Plant Cell Environ 29:1532–1544
Sandalio LM, Dalurzo HC, Gómez M, Romero-Puertas MC, del Río LA (2001) Cadmium-induced changes in the growth and oxidative metabolism of pea plants. J Exp Bot 52:2115–2126
Singh HP, Batish DR, Kaur G, Arora K, Kohli RK (2008) Nitric oxide (as sodium nitroprusside) supplementation ameliorates Cd toxicity in hydroponically grown wheat roots. Environ Exp Bot 63:158–167
Tamás L, Dudíková J, Ďurčeková K, Huttová J, Mistrík I, Zelinová V (2007) The impact of heavy metals on the activity of some enzymes along the barley root. Environ Exp Bot 62:86–91
Tewari RK, Hahn EJ, Paek KY (2008) Modulation of copper toxicity-induced oxidative damage by nitric oxide supply in the adventitious roots of Panax ginseng. Plant Cell Rep 27:171–181
Tian QY, Sun DH, Zhao MG, Zhang WH (2007) Inhibition of nitric oxide synthase (NOS) underlies aluminum-induced inhibition of root elongation in Hibiscus moscheutos. New Phytol 174:322–331
Valderrama R, Corpas FJ, Carreras A, Fernández-Ocaña A, Chaki M, Luque F, Gómez-Rodríguez MV, Colmenero-Varea P, del Río LA, Barroso JB (2007) Nitrosative stress in plants. FEBS Lett 581:453–461
Wang YS, Yang ZM (2005) Nitric oxide reduces aluminum toxicity by preventing oxidative stress in the root of Cassia tora L. Plant Cell Physiol 46:1915–1923
Xu M, Zhu L, Shou HX, Wu P (2005) A PIN1 family gene, OsPIN1, involved in auxin-dependent adventitious root emergence and tillering in rice. Plant Cell Physiol 46:1674–1681
Yang JL, Li YY, Zhang YJ, Zhang SS, Wu YR, Wu P, Zheng SJ (2008) Cell wall polysaccharides are specifically involved in the exclusion of aluminum from the rice root apex. Plant Physiol 146:602–611
Yeh CM, Chien PS, Huang HJ (2007) Distinct signalling pathways for induction of MAP kinase activities by cadmium and copper in rice roots. J Exp Bot 58:659–671
Zeidler D, Zähringer U, Gerber I, Dubery I, Hartung T, Bors W, Hutzler P, Durner J (2004) Innate immunity in Arabidopsis thaliana: lipopolysaccharides activate nitric oxide synthase (NOS) and induce defense genes. Proc Natl Acad Sci USA 101:15811–15816
Zhao DY, Tian QY, Li YH, Zhang WH (2007) Nitric oxide is involved in nitrate-induced inhibition of root elongation in Zea mays. Ann Bot (Lond) 100:497–503
Acknowledgments
The research was supported by the National Nature Science Foundation (No: 30671255), National Key Technology Research and Development Programme (No: 2006BAK02A18) and Project of National Key Basic Research and Development (2002CB410804) of China. We thank Dr Mosun Chen for technical assistants with microscopic analysis of CR primordia.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xiong, J., Lu, H., Lu, K. et al. Cadmium decreases crown root number by decreasing endogenous nitric oxide, which is indispensable for crown root primordia initiation in rice seedlings. Planta 230, 599–610 (2009). https://doi.org/10.1007/s00425-009-0970-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-009-0970-y