Abstract
Nitric oxide (NO) is recognized as an endogenous signaling molecule that plays an important role in the defence responses of medicinal plants to NaCl stress. In this study, we investigated the effects of sodium nitroprusside (SNP) as an NO donor at three concentrations (0, 100, and 200 µmol l−1) to alleviate the deleterious effects of salt stress (100 mM NaCl) on leaf gas exchange and biochemical characteristics of Silybum marianum L. seedlings. This study showed that salt stress significantly decreased relative water content (RWC), chlorophyll b content, endogenous NO concentration, maximum quantum yield (Fv/Fm), leaf gas exchange, stomatal size, K+/Na+ ratio, and plant dry weight, and increased malondialdehyde (MDA) content, hydrogen peroxide (H2O2) content, proline content, stomatal density, and enzyme activities. SNP treatment increased Fv/Fm, photosynthetic pigments, K+/Na+ ratio, and dry weights of the shoots and roots of NaCl-exposed plants. The exogenous application of NO increased the proline content under salinity stress more than under stress conditions without SNP application, so that the proline content increased from 32 to 47 µmol g−1. Application of 100 µM SNP also increased endogenous NO concentration (up to 43%) and consequently protected plants against salt stress-induced damage by improving enzyme activity and reducing the H2O2 generation rate (up to 14%) and MDA content (up to 50%) compared to plants treated with NaCl alone. Foliar application of NO to salt-stressed plants increased root and shoot respiration rates from 20 and 12%, respectively, under salinity stress to 57% under the application of SNP and stress conditions, and decreased stomatal conductance by up to 70%, resulting in improved RWC. Increased internal NO generation in plants induced by 100 µM SNP application has the potential to mitigate salinity injury in Silybum marianum L. plants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Salinity is a major environmental threat to plant growth and development and has become a serious problem in arid and semi-arid regions owing to global climate change in the last decade (Gupta et al. 2016; Karthik et al. 2019). Salinity has destroyed a large area of land (approximately 800 million hectares) worldwide, especially in Asia, creating a need to study plant tolerance to salinity and to provide strategies to mitigate salinity stress (Liu and Wang; 2021; Munns and Tester 2008). The consequences of salt stress include oxidative stress caused by the production of reactive oxygen species (ROS), which increases lipid peroxidation and causes damage to cells (Aghajanlou et al. 2021; Kumar et al. 2020). Other important effects of salinity stress include water deficit, altered K+/Na+ ratio, reduced uptake of essential elements, and many morphological, physiological, and metabolic changes, such as inhibition of various enzyme activities (such as Rubisco) and death at higher salt concentrations (Mahdavi et al. 2020; Rad et al. 2020; Ramadan et al. 2019). Salinity immediately affects stomatal conductance, which is caused by disturbed plant–water relations and local abscisic acid (ABA) synthesis (Fricke et al. 2004). In addition, higher salt accumulation induces oxidative stress in plants and increases cytosolic Na+ concentration, which causes electrolyte leakage and ultimately reduces plant growth (Ahmad et al. 2016; Ramadan et al. 2019). It has been researched that crop plants use K+, rather than Na+, as a vital component of osmotic adjustment and an essential macronutrient (Zhao et al. 2007). Research has shown that a decrease in K+ concentration and an increase in Na+ concentration in plants under salt stress can reduce the synthesis of important metabolic processes, such as photosynthesis and nutrient transport. All of these factors can lead to reduced plant growth (Yang et al. 2020; Liu and Wang; 2021). High concentrations of Na+ in the leaves are not desirable and disrupt stomatal movement. In plants under salinity stress, a decrease in stomatal cell turgor can reduce RWC content, leading to a decrease in K+ content and an increase in abscisic acid synthesis (Morant-Manceau et al. 2004). Salinity stress directly and indirectly affects leaf chlorophyll content and photosynthetic efficiency. These effects are achieved directly by regulating the activity of enzymes involved in chlorophyll synthesis, and indirectly by regulating the pathways involved in the antioxidant enzyme (Yang et al. 2020). Recently, the application of chemical compounds, such as sodium nitroprusside (SNP), a nitric oxide (NO) donor, as a novel strategy has improved plant growth and resistance to environmental stresses, such as drought, salinity, heavy metals, and plant diseases (Gupta et al. 2016; Mohasseli and Sadeghi 2019; Singh et al. 2017). Sodium nitroprusside serves as a signaling molecule in various plant species, regulating numerous physiological and metabolic responses and enhancing plant growth and performance under various stresses (Mohasseli and Sadeghi 2019; Karthik et al. 2019). Nitric oxide is an endogenous gas messenger that plays many roles in physiological processes such as stomatal closure, proline metabolism, ROS metabolism, leaf water maintenance, enhancement of antioxidant mechanisms, and reduction of hydrogen peroxide (H2O2) production and accumulation (Karthik et al. 2019; Li et al. 2008; Mostofa et al. 2015; Nabi et al. 2019; Ramadan et al. 2019; Zangani et al. 2021a). The application of NO donors typically strengthens plants against future stress, either by inducing antioxidant machinery or by stimulating the production of endogenous NO (Santisree et al. 2015). More recently, NO has been shown to be an elicitor involved in alleviating the effects of biotic and abiotic stresses on medicinal plants and regulating plant secondary metabolism (Akram et al. 2017; Du et al. 2015; Zangani et al. 2018, 2021b). Our previous study showed that NO can improve the yield of pharmaceutically active compounds in S. marianum under drought stress (Zangani et al. 2021). Studies have shown that the application of SNP can mitigate the damage caused by salinity by increasing the K+/Na+ ratio, which is induced by the activities of proton pumps and antioxidant enzymes (Ahmad et al. 2016; Liu et al. 2013). Furthermore, SNP application reduced hydrogen peroxide, lipid peroxidation, and sodium accumulation and improved the growth of rapeseed plants under saline conditions (Farouk and Arafa 2018).
Silybum marianum L. (S. marianum) is an annual/biennial medicinal plant of the Asteraceae family that is used to treat kidney, spleen, liver, and gallbladder diseases (Abenavoli et al. 2018; Karkanis et al. 2011). It is native to western and central Europe and northern India, and has been widely cultivated worldwide in recent years (Abenavoli et al. 2018). The economic and medicinal importance of S. marianum is mainly attributed to its accumulation of oil and a complex flavonolignan known as silymarin (Abenavoli et al. 2018; Zangani et al. 2021b). Furthermore, S. marianum is considered a pharmaceutical crop with low energy input and high economic value because of its high performance in arid and semiarid regions (Keshavarz Afshar et al. 2015; Zangani et al. 2018).
Considering the important role of NO in plant physiological and biochemical responses to abiotic stress, there is limited information in the literature on the response of S. marianum to exogenous application of SNP under salinity stress. In this study, we hypothesized that SNP would increase the salinity tolerance of S. marianum by activating biochemical processes and antioxidant enzymes. Therefore, the aim of this study was to investigate the possible impact of exogenous NO on alleviating the negative effects of NaCl stress on gas exchange and biochemical attributes, and consequently on the performance of S. marianum seedlings, as well as to alter plant respiration under salt stress.
Materials and Methods
Experimental Design, Plant Material, and Growth Conditions
The experiment was conducted at the Department of Plant Production and Genetics, University of Zanjan, Zanjan, Iran, in a factorial arrangement based on a randomized complete block design with three replicates. Seedlings were grown in a growth chamber with a day/night cycle of 16/8 h a temperature of 25/20 °C, relative humidity of 70%, and light intensity of 300 µmol photons m−2 s−1. The treatments consisted of salinity stress at two levels (0 and 100 mM NaCl) and SNP (as an NO donor) at three levels (0, 100, and 200 µmol l−1). The lethal level of salinity was normalized to a 100 mmol/l NaCl solution based on our initial experiment on the response of S. marianum seedlings to 60 and 120 mmol/l NaCl, as well as on previous studies on S. marianum ecotypes (Zahra et al., 2021; Ghavami and Ramin, 2008). The concentration range of SNPs in this experiment was chosen based on the literature for S. marianum and similar plants (Zangani et al. 2018; Fan and Liu 2011; Xu et al., 2005).
The experimental culture medium used in this study was washed with sand. The sand was autoclaved to remove microbial agents. S. marianum seeds (Sari genotype) were surface-sterilized with 5% sodium hypochlorite for 10 min and then washed twice with double-distilled water. Five seeds were sown in plastic pots (10 cm diameter × 15 cm depth) filled with 1200 g sand. Ten days after emergence, seedlings were thinned until three identical plants were maintained in each pot. During the growth period, seedlings were watered and fertilized every 2 d with nutrient solution containing 3 mM KNO3, 4 mM Ca(NO3)2, 2 mM MgSO4, 2 mM KH2PO4, 1 µM H3BO3, 0.4 µM MnSO4, 0.3 µM CuSO4, 0.06 µM H2MoO4, 2 µM ZnSO4, and 100 µM Fe-EDTA.
Fourteen days after seedling emergence, 100 mM NaCl dissolved in the nutrient solution was applied to the treatments. For exogenous NO application, the SNP (29.8 mg l−1 or 59.6 mg l−1 SNP for 100 or 200 µM treatments) was sprayed on the whole plant 21 days after emergence (7 days after salt application) at an interval of three days for 9 days. After the treatments (salt and SNP application), physiological measurements were performed on fully expanded leaves 30–35 days after emergence, and then the leaves of the plants were randomly sampled and stored at −80 °C for evaluation of physiological and biochemical traits.
Determination of Leaf Relative Water Content
Young, expanded leaves were immediately weighed to determine fresh weight (Fw) according to the method described by Siddique et al. (Machado and Paulsen 2001). The leaves were then immersed in deionized water at 4 °C for 8 h to determine the turgid weight (Tw). Finally, the dry weight of the leaves (DW) was obtained after drying the leaves in an oven at 75 °C for 24 h. The relative water content (RWC) of the leaves was calculated as follows:
Photosynthetic Pigment Content
Chlorophyll and carotenoid content were measured according to the methods of Arnon (1949) and Lichtenthaler (1987), respectively, using a UV/VIS spectrophotometer (PerkinElmer, Lambada 25, USA). Specifically, 100 mg of fresh leaves was ground in a mortar, and the obtained powder was homogenized in 80% (v/v) acetone. The homogenate was centrifuged at 6000 g for 10 min until the supernatant became clear. The absorbance of each sample was recorded at 663, 645, and 470 nm and calculated using the following formula: Photosynthetic pigments were expressed as mg g−1 fresh weight.
In the above formulas, A, V, and W represent the solution absorption rate, the final volume of the solution, and leaf fresh weight, respectively.
Determination of Free Proline
The leaf proline content was determined using the method described by Bates and colleagues (Bates et al. (1973)). Leaves (0.25 g) were ground in 5 ml of 3% sulfosalicylic acid solution using a mortar, and the resulting mixture was centrifuged at 3000×g for 10 min. Two ml of the supernatant was then mixed with 2 ml of acidic ninhydrin and 2 ml of glacial acetic acid in a test tube. The mixture was then heated in a water bath at 100 °C for 1 h. The mixture was then cooled on ice. It was extracted with 4 ml of toluene and shaken vigorously. The absorbance of the top layer was measured at 520 nm by using a UV/Vis spectrophotometer. The proline content was calculated from a calibration curve of the proline standard and expressed as µmol g−1 fresh weight.
Electrolytic Leakage
Electrolyte leakage was measured according to the method described by Lutts (1996). In this method, fresh leaf samples were cut into small pieces and incubated for 24 h at 25 °C in test tubes containing 10 ml of double-distilled water to measure the initial electrical conductivity (EC1) using a conductivity meter. The samples were then placed in a water bath at 100 °C for 30 min to measure the second electrical conductivity (EC2) at room temperature. Electrolytic leakage was calculated as a percentage, using the following formula:
Determination of Malondialdehyde (MDA)
Lipid peroxidation was determined by measuring the degree of oxidation of membrane fatty acids to malondialdehyde (MDA), as described by Du and Bramlage (2002). Leaf samples (250 mg) were ground in 5 ml of 0.1% (w/v) trichloroacetic acid using a mortar and pestle, and the homogenate was centrifuged at 3000×g for 10 min. One milliliter of the supernatant was added to 4 ml of 20% trichloroacetic acid containing 0.5% (w/v) thiobarbituric acid. The solution was incubated in a boiling water bath for 30 min, and then cooled on ice. The extract was centrifuged again at 3000×g for 5 min and the absorbance of the mixture was recorded at 440, 532, and 600 nm using a UV/VIS spectrophotometer (PerkinElmer, Lambada 25, USA). MDA levels were expressed as mmol g−1 fresh weight using an extinction coefficient of 155 mM−1 cm−1. The MDA concentration was calculated using the following equation:
where 532 nm is the maximum absorbance of the TBA-MDA complex, 600 nm is the correction for nonspecific turbidity, and 440 nm is the correction for the absorbance of sucrose, which approximates the total soluble sugars present in the samples.
Hydrogen Peroxide (H2O2)
The H2O2 content was measured according to the method described by Alexieva et al. (2001). Fresh leaf material (0.25 g), previously macerated in liquid nitrogen, was homogenized with 5 ml of trichloroacetic acid (0.1%, w/v) using a mortar and pestle. The resulting mixture was centrifuged at 8000 × g for 10 min. The supernatant (0.5 ml of the supernatant was added to 0.5 ml of potassium phosphate buffer (100 mM, pH 7.0) and potassium iodide (2 ml, 1 M). The absorbance of the supernatant was measured at 390 nm.
Antioxidant Enzyme Activity Assay
For enzyme extraction, samples (100 mg of fresh S. marianum leaves) were frozen in liquid nitrogen and homogenized in 1 ml ice-cold derivatization solution containing 50 mM potassium phosphate buffer (pH 7), 2 mM ethylenediaminetetraacetic acid (EDTA), and 2% (w/v) polyvinylpyrrolidone-25 (PVP-25) (Nakano and Asada 1981). The homogenate was centrifuged at 14,000×g for 30 min at 4 °C (centrifuge, Hettich-MIKRO 220R), and the supernatant was collected for soluble protein, catalase (CAT), peroxidase (POX), and ascorbate peroxidase (APX) assays. Soluble proteins were determined using bovine serum albumin (BSA) at 595 nm using a UV/Vis spectrophotometer according to the method described by Bradford (1976). CAT activity was measured with a UV/VIS spectrophotometer at 240 nm for 1 min and expressed as µM min−1 mg−1 protein using the extinction coefficient (ɛ = 39.4 mM−1 cm−1) (Aebi 1984). The reaction mixture contained 3 ml K-phosphate buffer (50 mM, pH 7), 15 mM H2O2 (30%), and 50 µl of enzyme extract. POX activity was determined by guaiacol oxidation in a reaction mixture containing 3 ml K-phosphate buffer (50 mM, pH 7), 15 mM H2O2 (30%), 10 mM guaiacol, and 50 µl of enzyme extract. The increase in absorbance was recorded at 470 nm for 2 min using a UV/VIS spectrophotometer and expressed as µM min−1 mg−1 protein using the extinction coefficient (ɛ = 26.6 mM−1 cm−1) (Chance and Maehly 1955). The APX activity was determined by the method described by Nakano and Asada at 290 nm using a UV/VIS spectrophotometer. The reaction mixture contained 3 ml K-phosphate buffer (50 mM, pH 7), 0.2 mM H2O2 (30%), 0.1 mM EDTA, 1 mM ascorbate and 50 µl enzyme extract and was expressed as µM min−1 mg−1 protein using the extinction coefficient (ε = 2.8 mM−1 cm−1) (Nakano and Asada 1981).
Na+ and K+ Content
Potassium (K+) and sodium (Na+) contents were determined using a previously reported method (Banerjee and Prasad 2020). Dried roots and shoots (100 mg) were subjected to wet digestion and digested with 10 ml of digestion mixture [HClO4:HNO3:H2SO4 (5:1:1, v/v)] on a hot plate (120 °C) until the volume was reduced to 1 ml. The extract was then diluted with double-distilled water. K+ and Na+ concentrations in shoots and roots were measured using a flame photometer (Jenway, model PFP7/C, UK). The K+/Na+ ratio in the roots and shoots was calculated from the concentration of these ions in the roots and shoots.
Determination of Nitric Oxide Concentration
The endogenous NO content in fresh leaves was determined using the Griess reagent according to the method described by Zhou and colleagues with slight modifications (Zhou et al. (2005) with slight modifications. This method is based on the conversion of NO to nitrite via Griess reaction. A leaf sample (0.5 g) was extracted with 50 mM acetic acid buffer (pH 3.6) containing 4% zinc acetate, using a mortar and pestle on ice. The homogenates were centrifuged at 8000 g for 15 min at 4 °C. The supernatant was collected. The test tubes were washed with 1 ml extraction buffer and centrifuged again, as described above. The two supernatants were combined. After shaking, the mixture was filtered through a paper filter and the filtered solution was mixed with 1% Griess reagent (1.5 ml). The mixture was then incubated at room temperature for 30 min. The absorbance of NO was measured at 540 nm by using a UV/Vis spectrophotometer. Sodium nitrite solutions (0–120 µgml−1) were used as standards. The data were expressed as µmol g−1 FW.
Chlorophyll a Fluorescence Measurement
Chlorophyll fluorescence was measured in young S. marianum leaves in the upper third of the stem at 25 °C using a fluorometer (PAM-OS-30, Opi-Seinces. UK.). The leaves were kept in the dark for 30 min and the initial fluorescence (Fo) and maximum fluorescence (Fm) were measured after exposure to saturated white light. The maximum photochemical efficiency of PSII (Fv/Fm) was estimated from the ratio of variable fluorescence (Fv) (Fv = Fm − Fo) to Fm in the dark-adapted leaves.
Root Respiration Measurement
Soil respiration refers to root, rhizosphere, and soil microbial respiration (Mohammadi et al. 2017; Xu et al. 2004). Furthermore, Yu and colleagues found that root respiration accounts for up to 60–70% of soil respiration (Yu et al. 2015). In this study, we used autoclaved sand to grow S. marianum; therefore, CO2 production in this study included root-derived CO2, which is a combination of plant root respiration and rhizosphere respiration. CO2 production was measured in a closed chamber (5 cm wide and 5 cm high) placed on the soil surface of the pot in a growth chamber under constant conditions (8 h of darkness at 20 °C, 16 h of light at 25 °C, and 70% relative humidity) using a Lambda device (Lambda, ADC Company, UK) (Mohammadi et al. 2017). The chamber was placed 1 cm below the sand in each pot and CO2 accumulation was measured after 30 min in the chamber. Three pots were prepared without plants, and respiration was considered rhizosphere respiration. Finally, the root respiration (μmol m−3 s−1) was determined as follows:
Shoot Respiration Measurement
To measure whole-plant respiration of S. marianum, the pot containing the plant was placed in a closed system (30 cm wide and 30 cm high) at a constant temperature (25 °C) under dark conditions, and the initial CO2 concentration in the chamber was measured using a Lambda device. Measurements of night respiration were performed after 30 min of acclimation to darkness and the accumulated CO2 in the closed chamber was measured. The surface of the pot was covered with aluminum foil to avoid interference between root and shoot respiration. Finally, shoot respiration (μmol m−3 s−1) was determined as follows:
Shoot respiration = Accumulated CO2 in the chamber after 30 min of initial CO2 concentration in the chamber.
Stomatal Conductance Measurement
The stomatal conductance was measured using a portable porometer (AP4, Delta-T Devices, UK). Readings were obtained from three fully expanded leaves in the upper third of the stem and were expressed as mmol m−2 s−1.
Stomatal Characteristics
The length, width, and density of stomata in the upper and lower epidermis of the leaves were evaluated using previously reported methods (Radoglou and Jarvis 1990; Malone et al. 1993). The youngest mature leaves were selected for analysis of stomatal characteristics. The leaf surface was first wiped with a clean cotton pad, and then smeared with a thin layer of transparent liquid glue. The thin layer was separated from the leaf surface, fixed on a glass slide, and examined for the number of stomata using a light microscope (Leica Galen ΙΙΙ model, USA). Stomatal density was expressed as the number of stomata per mm2. The stomatal length and width were measured in micrometers and expressed in micrometers.
Shoot and Root Dry Weight
To measure root and shoot dry weights, plants were harvested at the end of the experiment (i.e., 45-day-old plants) and divided into roots and shoots. The roots were washed twice to remove excess salt. Their Dry weights were measured after drying in an oven at 75 °C for 48 h.
Statistical Analysis
A two-way factorial (2 × 3) completely randomized design with three replications was used for data analysis. The first factor was the two salt levels and the second factor was the three SNP concentrations. SAS statistical software (SAS, Institute Inc. 2009) was used for analysis of variance (ANOVA). When the effects were significant (P ≤ 0.05), differences between means were evaluated using Duncan's test (P ≤ 0.05).
Results
Relative Water Content (RWC)
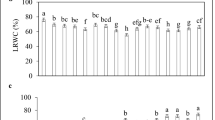
The interaction between SNP and salt stress in RWC was significant (P ≤ 0.05). Salt stress induced a significant 30% reduction in the RWC of S. marianum leaves compared to that of the control plants. Under salt stress conditions, RWC increased by up to 10.4% when plants were sprayed with 100 µM SNP compared to non-sprayed plants (Fig. 1A).
Effects of SNP application on leaf RWC (A) and proline content (B) of S. marianum under salinity stress. Data are presented from the two-way factorial according to the significance of ANOVA. Data are expressed as mean ± standard error (SE). Different letters at the top of the bars indicate significant differences at P < 0.05 by Duncan. Vertical bars indicate SE. Control is non-salt stress
Free Proline Content
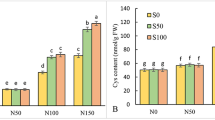
The effects of SNP and salt stress and the interaction of SNP × salt stress were significant for leaf proline content (P ≤ 0.01). The lowest proline concentration was observed in the control plants (no salt stress). Conversely, plants exposed to salt stress exhibited an increased proline content. Under salt stress conditions, a further increase in proline accumulation (up to 47%) was observed in response to the application of 100 μM SNP compared with the non-SNP treatment (Fig. 1B).
Leaf Electrolyte Leakage
The effects of SNP and salt stress on leaf EL were significant (P ≤ 0.01). However, no significant differences were observed in the interactions between the SNP and salt stress. Electrolyte leakage in S. marianum leaves was significantly increased by salt stress, by up to 90.7% (Fig. 2). In contrast, the exogenous application of 100 µM SNP reduced electrolyte leakage by 27% compared to the non-application of SNP (Fig. 2).
Effects of salt stress and SNP application on leaf electrolyte leakage of S. marianum. Control, no salt + SNP; salt stress, salt + SNP; non-SNP, no SNP + control or salt; SNP100 and SNP200, SNP100 or 200 + control or salt. Data are presented from the two-way factorial scheme according to the significance of ANOVA. Data are expressed as mean ± standard error (SE). Different letters at the top of the bars indicate significant differences at P < 0.05 by Duncan's signed rank test. Vertical bars indicate SE
MDA Content and H2O2 Generation Rate
The effects of SNP and salt stress and the interaction of SNP × salt stress were significant for MDA and H2O2 levels in S. marianum leaves (P ≤ 0.01). In the salt treatment, MDA and H2O2 concentrations increased by 102% (Fig. 3a) and 69% (Fig. 3b), respectively, compared with the control treatment. In contrast, plants treated with 100 µM SNP showed a significant decrease in MDA (up to 50%) and H2O2 (up to 14%) levels under salt stress compared with plants exposed to salt stress without SNP (Fig. 3A and B). In addition, exogenous application of 200 µM SNP caused a non-significant decrease in MDA (up to 17%) and H2O2 (up to 4.6%) levels under salt stress (Fig. 3A and B).
Effects of SNP application on leaf MDA content (A) and leaf H2O2 content (B) of S. marianum under salt stress. Data are presented from the two-way factorial scheme according to the significance of ANOVA. Data are expressed as mean ± standard error (SE). Different letters at the top of the bars indicate significant differences at P < 0.05 by Duncan. Vertical bars indicate SE
Photosynthetic Pigment Content
Salt stress decreased chlorophyll b (Fig. 4B), total chlorophyll (Fig. 4C), and carotenoid (Fig. 4D) content by up to 25, 14, and 16%, respectively, compared to control plants. Under salt stress, both SNP concentrations increased chlorophyll a (Fig. 4A), chlorophyll b, total chlorophyll, and carotenoids compared to the non-SNP, with the 100 µM SNP showing the highest increase of up to 29, 48, 40, and 43%, respectively (Fig. 4).
Effects of SNP application on chlorophyll a (A), chlorophyll b (B), total chlorophyll (C) and carotenoid content (D) in leaves of S. marianum under salt stress. Data are presented from the two-way factorial scheme according to the significance of ANOVA. Data are expressed as mean ± standard error (SE). Different letters at the top of the bars in each trait indicate significant differences at P < 0.05 by Duncan. Vertical bars indicate SE
Enzyme Activity
The results showed that salt stress increased APX, CAT, and POX activities by up to 39%, 189%, and 52%, respectively, compared to the control (non-stressed) seedlings of S. marianum (Fig. 5). CAT activity was increased by the application of 100 µM SNP (up to 37%) and decreased by the application of 200 µM SNP (up to 29%) compared with non-SNP under salt stress (Fig. 5A). In contrast, under salt stress, POX activity was increased by the application of 100 µM SNP (up to 49%) and decreased by the application of 200 µM SNP (up to 38%) compared with non-SNP (Fig. 4B). Under salt stress, 100 and 200 µM SNP caused a decrease in APX activity by up to 31 and 29%, respectively, compared to non-SNP (Fig. 5C).
Effects of SNP application on the activities of catalase (CAT) (A), peroxidase (POX) (B), and ascorbate peroxidase (APX) (C) in S. marianum leaves under salt stress. Data are presented from the two-way factorial scheme according to the significance of ANOVA. Data are expressed as mean ± standard error (SE). Different letters at the top of the bars in each trait indicate significant differences at P < 0.05 by Duncan. Vertical bars indicate SE
Na+ and k+ Ions Changes Under Salt Stress
The results of K+ and Na+ content and Na+/K+ ratio showed that salt stress increased Na+ content in the roots and shoots and the Na+/K+ ratio in the roots and shoots of S. marianum by up to 25, 91, 33, and 137%, respectively, compared to the control plants. However, the Na+ content and Na+/K+ ratio in the shoots decreased by 6.2 and 21.7%, respectively, when the plants were treated with 100 µM SNP under salt stress. In contrast, 100 µM SNP and salt stress resulted in an increase in Na+ content and Na+/K+ ratio (up to 22 and 5.8%, respectively) in the roots of S. marianum compared to plants under salt stress without SNP (Table 1). However, exposure of plants to salt stress caused a 5 and 25% reduction in K+ content in the roots and shoots of S. marianum, respectively, compared to the control (non-stressed) plants. However, the K+ content of the roots and shoots increased by up to 15.2 and 21.6%, respectively, when plants were treated with 100 µM SNP under salt conditions (Table 1).
Nitric Oxide Concentration (NO)
The effects of SNP and salt stress and the interaction between SNP and salt level were significant for NO content in S. marianum plants (P ≤ 0.01). In general, the concentration of NO is altered by SNP and salt levels. Exposure of plants to salt stress caused a 40% decrease in endogenous NO concentration compared with the control (Fig. 6A). Exogenous application of 100 µM SNP resulted in a 43% increase in NO concentration compared to no SNP application under salt stress (Fig. 6A and Table 3). However, no increase in the NO concentration was observed when 200 µM SNP was applied under salt stress.
Effects of SNP application on leaf endogenous NO content (A) and PSII quantum yield (B) of S. marianum under salt stress. Data are presented from the two-way factorial according to the significance of ANOVA. Data are expressed as mean ± standard error (SE). Different letters at the top of the bars indicate significant differences at P < 0.05 by Duncan. Vertical bars indicate SE
Quantum Yield of Photosystem II
ANOVA results for the quantum yield of photosystem II (Fv/Fm) of S. marianum showed that there was no significant difference in the effects of SNP and salt stress, but the interaction effect of SNP × salt stress was significant for this trait (P ≤ 0.01). The results showed that exposure of S. marianum plants to 100 mM NaCl reduced the quantum yield of photosystem II by up to 10.6% compared with the control (non-salt stressed). Application of 100 µM SNP increased this by up to 9.2% compared with the non-application of SNP under salt stress (Fig. 6B). In contrast, the exogenous application of 200 µM SNP did not cause a significant increase (Fig. 6B).
Stomatal Conductance
The effects of SNP and salt stress and the interaction of SNP and salt stress on leaf stomatal conductance were significant (P ≤ 0.01). Leaf stomatal conductance was significantly decreased by salinity up to 75.4% and followed a decreasing trend with increasing SNP levels (Fig. 7). Under control (non-saline) conditions, the application of SNP at both concentrations decreased the leaf stomatal conductance. The lowest leaf stomatal conductance was obtained with the application of 100 µM SNP under the salinity treatment, which showed a significantly greater reduction (up to 69.6%) than those subjected to salinity stress without SNP (Fig. 7).
Effects of SNP application on leaf stomatal conductance of S. marianum under salt stress. Data are presented from the two-way factorial scheme according to the significance of ANOVA. Data are expressed as mean ± standard error (SE). Different letters at the top of the bars indicate significant differences at P < 0.05 by Duncan. Vertical bars indicate SE
Stomatal Characteristics
The interaction of SNP × salt stress on stomatal characteristics showed that salt stress decreased stomatal width in the lower epidermis of the leaf, stomatal width in the upper epidermis of the leaf, stomatal length in the lower epidermis, and stomatal length in the upper epidermis by up to 16, 9, 30, and 22%, respectively, compared to that in control (non-stressed) plants. However, the stomatal width in the lower epidermis, stomatal width in the upper epidermis, stomatal length in the lower epidermis, and stomatal length in the upper epidermis increased by 3.9, 13.4, 15.8, and 24.9%, respectively, when plants were treated with 100 µM SNP under stress conditions (Table 2). Under salt stress, stomatal density in the lower and upper epidermis of the leaf increased by 93 and 117%, respectively, compared with the control (non-stressed) plants. However, plants treated with SNP (especially at 100 µM concentration) and salt stress showed a decrease in stomatal density in the lower (up to 45%) and upper (up to 58%) epidermis of the leaves compared to plants exposed to salt stress without SNP (Table 2).
Root and Shoot Respiration
The effects of SNP and SNP × salt stress interactions on root and shoot respiration were significant. However, the effect of salt stress was significant only for shoot respiration (P ≤ 0.01). Salt stress significantly reduced root and shoot respiration by 20.6 and 12.7%, respectively (Fig. 8A, B). Under both non-stress and salt stress conditions, SNP concentrations increased root and shoot respiration. Under salt stress, root and shoot respiration increased significantly with SNP application (especially at 100 µM), up to 57.1 and 9.8%, respectively, compared with non-application of SNP. In addition, the application of 200 µM SNP increased shoot respiration by up to 57.1% compared to the non-application of SNP under stress conditions (Fig. 8B).
Effects of SNP application on root (A) and shoot (B) respiration of S. marianum under salt stress. Data are presented from the two-way factorial according to the significance of ANOVA. Data are expressed as mean ± standard error (SE). Different letters at the top of the bars in each trait indicate significant differences at P < 0.05 by Duncan. Vertical bars indicate SE
Growth Parameters
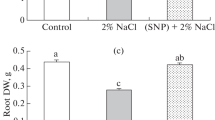
The root and shoot dry weights were reduced by up to 66 and 37%, respectively, under salt stress (Fig. 9A, B). Both SNP concentrations increased the root and shoot dry weights under both non-stressed and salt-stressed conditions. Root and shoot dry weights increased by up to 172 and 33%, respectively, when the plants were treated with 100 µM SNP under saline conditions (Fig. 9A, B).
Effects of SNP application on dry weight of S. marianum shoot (A) and root (B) under salt stress. Data are presented from the two-way factorial according to the significance of ANOVA. Data are expressed as mean ± standard error (SE). Different letters at the top of the bars in each trait indicate significant differences at P < 0.05 by Duncan. Vertical bars indicate SE
Discussion
Salt stress significantly inhibited the growth of S. marianum, which is consistent with previous reports on other crops (Ahmad et al. 2018; Ghassemi-Golezani and Lotfi 2015; Kaya et al. 2018; Ragaey et al. 2022; dos Santos et al. 2022). However, SNP supplementation reduced the detrimental effect of salinity on S. marianum plant growth, which is consistent with previous findings in different crops, such as rice, Solanum lycopersicum, and wheat (Gupta et al. 2016; Adamu et al. 2018; Ahmad et al. 2018; Ragaey et al. 2022). In many plants, salt stress perturbs water status and decreases leaf RWC (Ahmad et al. 2018; Karthik et al. 2019), which is consistent with our findings. Under salt stress conditions, root water uptake is challenged by the reduced osmotic potential of the root environment, and the amount of water in the leaf tissues and cells decreases. Consequently, RWC also decreases (dos Santos et al. 2022). A small decrease in stomatal conductance under salt stress may have a protective effect against stress by improving plant water use efficiency, as reported in other studies (Kelly et al. 2019; Liao et al. 2022). Under salt stress, the RWC increased with the application of 100 μM SNP (Fig. 6). NO increases the accumulation of osmotic regulators (e.g., proline), leading to maintenance and increase in plant RWC. This was observed in the studies by Ghadakchiasl et al. (2017) and El Moukhtari et al. (2020). Consequently, further stomatal closure (Table 2) and a further decrease in stomatal conductance correlated with a 10% increase in RWC under salt stress (Fig. 1A). Meanwhile, the accumulation of osmolytes due to SNP application may increase osmotic regulation, and consequently, the decreased osmotic potential may increase water uptake, and subsequently, RWC. These results were consistent with those reported by other authors (Garcia-Mata and Lamattina 2007; Sousa et al. 2019).
Salinity stress significantly reduced the pigment content in S. marianum plants, whereas the application of 100 µM SNP compensated for this reduction, as observed in other plants (Nasiri-Savadkoohi 2017; Shi et al. 2016; Yasir et al. 2021; Ragaey et al. 2022). Elevated levels of H2O2 damage thylakoid membranes, proteins, and chlorophyll. On the other hand, chloroplast disruption, activation of the chlorophyllase enzyme, and decreased uptake of micronutrients such as Fe may be the reasons for the decrease in chlorophyll content (Farouk and Arafa 2018; Liu and Shi 2010; Liu et al. 2013). In this study, the increase in chlorophyll content with SNP application could be attributed to the reduction of H2O2 with SNP application. Consistent with our results regarding the beneficial effects of SNP on chlorophyll, Rasheed et al. (2022) demonstrated that NO application increases chlorophyll content and decreases H2O2 production in plants under abiotic stress conditions. Shi et al. (2016) reported that SNP significantly inhibited the activities of chlorophyll-degrading enzymes such as chlorophyllase, chlorophyll-degrading peroxidase, Mg-dechelatase, and pheophytinase, and increased the concentrations of chlorophyll a and b (Shi et al. 2016). In addition, NO appears to increase plant access to iron to maintain chlorophyll content, and iron plays a major role in the structure of chloroplast protein units (Azizi et al. 2021).
Proline is a non-enzymatic antioxidant metabolite that also acts as an energy storage molecule under saline conditions, resulting in salt stress tolerance and ROS scavenging ability (Surender Reddy et al. 2015). Increased proline content in response to salt stress has been reported in Nitraria tangutorum (Liu et al. 2016), Linum usitatissimum (Khan et al. 2012), Oryza sativa (Adamu et al. 2018), and Morus alba (Ahmad et al. 2016), which is consistent with our findings. Because glutamate is a precursor for chlorophyll and proline synthesis, and chlorophyll content is reduced under stress conditions, nitrogen metabolism shifts from glutamate to proline synthesis as chlorophyll content decreases, leading to increased proline accumulation under salinity conditions (Yousefvand et al. 2022). Furthermore, proline content is negatively correlated with the total chlorophyll (Ghadakchiasl et al. 2017). However, 1-pyrroline-5-carboxylate synthetase activity and the glutamate pathway for proline biosynthesis increase upon salt stress treatment, and play a more significant role in proline accumulation during stress (El Moukhtari et al. 2020). Furthermore, application of 100 µM SNP accelerated proline accumulation, which was greater than that under salt stress alone (Fig. 1B). Consistent with our results regarding the effect of salinity and SNP on proline, an increase in proline content has been reported in both salinity and SNP treatments in wheat (Ragaey et al. 2022) and raspberry (Ghadakchiasl et al. 2017). It has been noted that arginine content may increase with SNP application of SNP (Ragaey et al. 2022). Another important source of NO is arginine, which is the main amino acid involved in proline production of proline (Liu et al. 2006). Salt stress tolerance can be induced by the application of NO, which can activate some key enzymes in the synthesis of proline and consequently increase its levels (Zhang et al. 2008; Liu et al. 2013; Ahmad et al. 2016). Recently, it has been claimed that exogenous NO can induce the P5CS1 gene, which encodes 1-pyrroline-5-carboxylate synthetase, a key enzyme involved in proline synthesis (Zhang et al. 2008; Ben Rejeb et al. 2014).
Accumulation of ROS, such as H2O2, under salt stress in plants degrades cell membrane lipids and consequently increases the MDA concentration and electrolyte leakage (Ahmad et al. 2016; Farouk and Arafa 2018; Kaya et al. 2018). In this study, salt stress increased H2O2 and MDA content and leaf electrolyte leakage, which may be due to membrane damage caused by salinity-induced ROS, which could inhibit photosynthesis and growth (Idrees et al. 2011; dos Santos et al. 2022). Under salinity conditions, increased Na+ content, lipid peroxidation, and damage to the plasma membrane can lead to the loss of selective permeability properties of the membrane and increased electrolyte leakage (Ghadakchiasl et al. 2017). In addition, the increase in electrolyte leakage may be related to an increase in chlorine and sodium entry, and a decrease in potassium uptake (Mohammadreza et al. 2012). Under salinity stress, foliar application of SNP at low concentrations as an effective H2O2 scavenger inhibited salinity-induced H2O2 production and consequently reduced MDA content and electrolyte leakage, which is consistent with other studies (Ahmad et al. 2018; Akram et al. 2017; Gupta et al. 2016; Ma et al. 2021). NO appears to stimulate mitogen-activated protein kinase (MAPK) to induce the expression of genes related to salt stress tolerance (Ahmad et al. 2016). Furthermore, NO can act on phospholipid bilayers to induce membrane fluidity by reducing membrane lipid peroxidation via antioxidant metabolites, which may reduce electrolyte leakage (Akram et al. 2017; Leshem and Haramaty 1996). In addition, NO can act as a free-radical scavenger and react with alcoholic lipid radicals and peroxide lipids, thereby directly inhibiting the lipid peroxidation chain. The role of NO in reducing membrane lipid peroxidation was reported by Ragaey et al. (2022).
In plant cells, ROS can cause oxidative damage and peroxidation of membrane lipids, resulting in leakage of cell membranes and damage to photosynthesis and chloroplast pigments (Ahmad et al. 2016). In contrast, cells can produce antioxidants (e.g., CAT and POX) that provide protection against oxidative damage (dos Santos et al. 2022). In the present study, salinity stress significantly increased APX activity in S. marianum, whereas 100 µM SNP treatment decreased it. Salt-stressed plants exhibited higher levels of CAT, POX, and APX activities, which could be involved in quenching ROS from stressed cells, limiting cellular damage, and improving the oxidative capacity of plants to protect against stress conditions. CAT and POX activity increased with 100 μM SNP and decreased with 200 μM SNP under salinity stress (Fig. 5A and B), which is consistent with the results of other studies (Shi et al. 2016; Nasiri-Savadkoohi 2017; Ragaey et al. 2022). In this study, the incremental effect of SNP on the antioxidant capacity of S. marianum plants could be due to the protective effect of released NO. In this regard, it has been reported that the increased CAT and POX activities could be due to the role of NO as a signaling molecule in stimulating the expression of their genes (Ahmad et al. 2018; Ma et al. 2021; Shi et al. 2016). Furthermore, the results of our study showed that the application of exogenous NO induced the synthesis of endogenous NO (Fig. 6A), which can act as a signaling molecule or ROS scavenger by increasing the activity of antioxidant enzymes under salt stress (Fan and Liu 2011; Liu et al. 2013). Therefore, external sources of NO, such as SNP, may induce internal NO generation with signaling or scavenging properties. The role of NO in ROS scavenging has been implicated in the repair of salinity stress in chloroplasts, which reduces carbohydrate synthesis (Wei et al. 2014). In addition, NO application increases salinity tolerance and improves the nutritional status of spinach by increasing CAT and POX activity, thus alleviating oxidative damage caused by salinity stress (Du et al. 2015). Another possible function of NO-induced salinity tolerance in S. marianum plants may be to promote antioxidant defense mechanisms to scavenge H2O2, resulting in higher chlorophyll content, similar to the findings of Ragaey et al. (2022).
One of the responses of plants to salt stress is a decrease in K+ and increase in Na+ in plant tissues. The accumulation of Na+ has negative effects on plant growth and development, and can lead to nutrient imbalances (Munns and Tester 2008; Liu et al. 2013). The accumulation of Na+ in cell organelles under salt stress causes depolarization of the plasma membrane and disrupts the ionic balance, resulting in an increase in the Na+/K+ ratio (Table 1) (Gupta et al. 2016; Kaya et al. 2018). The results of this study on the effect of salinity stress on the decrease in K+ and increase in Na+ and Na+/K+ ratios are consistent with the results of the above studies. Salinity stress increases the amount of sodium and chlorine and decreases the amount of potassium and calcium through ion homeostasis disorders (Naheed et al. 2021). The results of the current study showed that foliar application of 100 μM SNP prevented the transfer of Na+ to the shoot by increasing Na+ accumulation in the roots under saline conditions and instead transferred more K+ ions to the shoot, which significantly reduced the Na+/K+ ratio in the shoot compared to non-SNP application (Table 1). The increase in K+ content due to SNP application may be due to the competition between the two ions for transport on a carrier protein, resulting in the replacement of K+ with Na+, whereas Na+ passively diffuses into the cytosol of root cells through ion channels (Khan et al. 2012). Therefore, spraying SNP could limit Na+ uptake by inhibiting passive Na+ influx and improving ion transport from roots to shoots under salt stress (Liu et al. 2013). In addition, NO has been shown to enhances salt tolerance by regulating the Na+/K+ ratio, which depends on the enhanced expression of plasma membrane H+-ATPase, vacuolar H+-ATPase, and H+-PPase activities, thus facilitating Na+ compartmentation through the Na+/H+ antiporter under saline conditions (Molassiotis et al. 2014; Shi et al. 2016).
Some studies have shown that environmental stress increases NO concentration in plants (Xiong et al. 2011; da Silva et al. 2017; Ma et al. 2021). In the case of salt stress, NO generation is transient (Zhang et al. 2006). Here, we confirmed that salt stress significantly reduces endogenous NO levels in S. marianum. In plants, NO is enzymatically produced from NO2 − by NAD(P)H-dependent nitrate reductase (Wendehenne et al. 2001). Researchers have attributed the reduction in endogenous NO by salinity to the reduction in nitrate reductase activity under salinity-stress conditions (Ragaey et al. 2022). In addition, the exogenous application of 100 μM SNP increased endogenous NO production under conditions of excess salinity, as previously reported (Fan and Liu 2011; Batista et al. 2018). It has been suggested that exogenous NO may allow the compound to rapidly cross membranes and activate signal transduction, thereby inducing NO synthesis pathways in plants (Gupta et al. 2016). Therefore, the application of exogenous NO sources such as SNP in plants may increase endogenous NO biosynthesis and enzyme activity through MAPK and other unknown pathways (Ahmad et al. 2016; Ragaey et al. 2022). It has also been reported that nitrate reductase activity is induced by the application of NO to plants, which may be reflected in the internal NO content (Ragaey et al. 2022).
The Fv/Fm ratio, which indicates the maximum quantum efficiency of PSII, is used as a measure of the rate of linear electron transport and an indicator of the overall photosynthetic capacity (Liu and Shi 2010; Singh-Tomar et al. 2012). Under salinity stress, electron transport in the PSII reaction center is disrupted and PSII performance and function are reduced (Kalaji et al. 2016). A decrease in the maximum quantum yield of PSII under salinity stress has been reported in cotton (Liu et al. 2013) and mung beans (Ghassemi-Golezani and Lotfi 2015), which was also observed in this study and may be related to the lower chlorophyll b and carotenoid levels (Fig. 4B and D). However, foliar application of SNP had a positive and alleviating effect on the maximum quantum yield of PSII (Fv/Fm) in S. marianum leaves under saline conditions (Fig. 6B). The inductive effect of SNP-generated NO on PSII photochemistry appears to be related to a promoter in the open PSII reaction centers, which can reduce the number of inactive reaction centers and lead to an increase in the initial fluorescence (F0) (Singh-Tomar et al. 2012). Therefore, NO application directly affects the electron transport chain in chloroplasts (Wodala et al. 2008). In addition, the enhancement of enzyme activities and pigment content, as well as the increase in K+ and decrease in Na+ accumulation by the application of SNP under saline conditions, can reduce enzyme inhibition and membrane disorganization (Munns and Tester 2008), as a result, PSII activity can be maintained and improved at a high level (Liu et al. 2013). The application of NO can improve photosystem efficiency, increase chlorophyll content, and reduce free radicals in plants under abiotic stress conditions (Rasheed et al. 2022).
In this experiment, salinity stress significantly increased the number of stomata in both the upper and lower epidermis of the leaves and decreased stomatal dimensions. The results showed that Stomatal length (Table 2) had the greatest effect on stomatal closure. However, SNP application prevented an increase in stomatal number under salinity stress and resulted in a smaller decrease in stomatal dimensions than non-SNP application under salinity stress (Table 2). Therefore, the greater and more significant reduction in stomatal conductance under salinity stress with 100 μM SNP application under these conditions can be attributed primarily to a 50% reduction in stomatal number, and then to the reduction in stomatal dimensions. Under salinity stress without SNP application, stomatal number increased simultaneously with a decrease in stomatal dimensions. Most studies have demonstrated the ability of NO to reduce the stomatal aperture (Sami et al. 2018; Santisree et al. 2015), which is involved in gas exchange. Changes in stomatal conductance are induced by ROS, NO, H2O2, hormones, Ca+2, and kinase proteins (Arasimowicz-Jelonek et al. 2009). Studies have shown that NO releases Ca+2 signaling molecules in guard cells, which regulate K+ and Cl− channels in the plasma membrane and induce stomatal closure (Garcia-Mata and Lamattina 2007; Mioto and Mercier 2013). Thus, the maximum stomatal conductance can be determined by the morphological characteristics of the stomata, such as stomatal size and density (GalmÉS et al. 2013). Therefore, although NO has only been shown to be involved in the control of stomatal opening and closing (Sun et al. 2017), the results of this study showed that NO is involved in the development and formation of guard cells under salt stress conditions, which may affect stomatal distribution in the leaf or conserve leaf water as leaf trichomes (Ning et al. 2016). These results are consistent with those of previous studies on Arabidopsis (Fu et al. 2016) and soybean (Sousa et al. 2019).
In the present study, the reduction in stomatal conductance under salt stress resulted in a reduction in CO2 derived from respiration (Fig. 8A and B), which may be due to the lack of change in mesophilic capacity. However, Tang (2002) stated that CO2 depletion could be responsible for a reduction in gas exchange (Tang 2002). Thus, salt stress reduces stomatal conductance to minimize water loss, which affects CO2 assimilation (Morant-Manceau et al. 2004). By reducing the dimensions of the stomata, salt exposure causes stomatal closure, which reduces the rate of photosynthesis owing to a decrease in stomatal conductance, thus limiting the access of CO2 to the Calvin-Benson cycle (dos Santos et al., 2022). In the present study, a reduction in plant respiration was observed under saline conditions, which was consistent with the findings of Anjum (2011). Reduced photosynthetic rates are likely to decrease the supply of substrate to the mitochondria, leading to a lower rate of foliar respiration (dos Santos et al. 2022). In contrast, root and shoot respiration increased with external application of NO under stress conditions, whereas stomatal conductance decreased. The application of SNP at an appropriate concentration plays an important role in scavenging ROS by compensating for internal NO reduction under stress conditions, which improves plant respiration, particularly root respiration. Some studies have shown that adaxial stomatal adjustment reduces transpiration, whereas the theoretical maximum abaxial stomatal conductance affects photosynthesis process (Sousa et al. 2019). The relationship between stomatal distribution and theoretical maximum stomatal conductance showed an equilibrium between transpiration and respiration, resulting in a higher respiration rate in plants sprayed with SNP. Hayat and colleagues reported that the application of SNP in tomato plants increased carbonic anhydrase activity, which catalyses the conversion of CO2 to HCO3 and the constant supply of CO2 to RuBisCO (Hayat et al. 2011).
The present study also showed that increasing internal NO in plants with SNP application had a greater effect on increasing root respiration (57%) than shoot respiration (13%) in S. marianum under stress conditions (Table 3). We hypothesized that internal NO affects stomatal structure and causes stomatal closure, resulting in a greater reduction in stomatal conductance compared with salinity conditions without SNP. As a result, by closing the stomata and reducing stomatal conductance, shoot respiration (assessed by examining the amount of CO2 released from stomata) was less affected by increased internal NO than root respiration under stress conditions (Table 3). Dry weight loss caused by salinity stress may be due to a decrease in RWC, chlorophyll content, photosynthesis, nutrient uptake, and an increase in electrolyte leakage, H2O2, MDA, and Na+ ions, and consequently oxidative stress. Salt accumulation in leaves leads to damage to chloroplasts and photosynthetic processes, osmotic effects, and reduced cell division and elongation, which are the main causes of dry weight loss (Farouk and Arafa 2018). In some plants, an increase in shoot dry weight after SNP application under salinity stress has been reported (Farouk and Arafa 2018; Yasir et al. 2021). As shown in the results, under salinity stress conditions, foliar application of 200 μM SNP decreased MDA (Fig. 3A), H2O2 (Fig. 3B), electrolyte leakage (Fig. 2), and stomatal conductance (Fig. 7), and increased RWC (Fig. 1A), chlorophyll (Fig. 4), carotenoids (Fig. 4D), and quantum yield (Fig. 6B) compared to non-application of SNP. All these parameters increased dry weight (Fig. 9) with 200 μM SNP treatment. However, the beneficial effect of 200 μM SNP was less than that of 100 μM SNP alone. In our study, SNP increased the activity of antioxidant enzymes as well as the levels of proline, RWC, chlorophyll, and K+ ions under salt stress, which resulted in improved plant growth. These results were similar to those of previous studies (Ghadakchiasl et al. 2017; Ragaey et al. 2022). It has been reported that the improved effect of SNP at a lower concentration on dry weight could be due to an increase in photosynthesis by increasing chlorophyll and carotenoid content, a decrease in oxidative stress by reducing H2O2, and protection of cell membranes from peroxidation by decreasing MDA as an indicator (Farouk and Arafa 2018; Nasiri-Savadkoohi 2017). However, the improvement in plant growth could be due to the potential role of NO in cytokinin signaling in plants (Ghadakchiasl et al. 2017).
Conclusions
The present study shows that exogenous NO could be useful for improving the growth and biochemical characteristics of S. marianum under normal or saline conditions. Foliar application of SNP to NaCl-stressed plants showed that NO improved salt tolerance by increasing the activities of antioxidant enzymes, RWC, proline, pigment content, and quantum yield of photosystem II, decreasing the H2O2 generation rate and membrane lipid peroxidation, and consequently maintaining a lower Na+/K+ ratio. This attenuation was associated with an increase in endogenous NO via the application of exogenous NO donors, which increased the performance of salt-stressed plants. Based on the results of the current study, leaf gas exchange and stomatal dimensions decreased under salinity stress, and the most significant decrease was related to leaf stomatal conductance and shoot respiration, suggesting that the effect of stress on the photosynthetic processes of the plant was greater than that on respiration. In contrast, stomatal conductance decreased with the external application of SNP under salt stress, which was attributed to a reduction in stomatal number, while root and shoot respiration increased. In conclusion, exogenous NO plays an important protective role in salt-stressed S. marianum plants, and 100 µM SNP under salinity stress had more beneficial effects on the salinity tolerance of S. marianum than did 200 µM SNP.
Data Availability
All data generated or analyzed during this study are included in this published article and are available upon request from the corresponding author.
Abbreviations
- APX:
-
Ascorbate peroxidase
- CAT:
-
Catalase
- H2O2 :
-
Hydrogen peroxide
- MDA:
-
Malondialdehyde
- NO:
-
Nitric oxide
- POX:
-
Peroxidase
- ROS:
-
Reactive oxygen species
- RWC:
-
Relative water content
- SNP:
-
Sodium nitroprusside
References
Abenavoli L, Izzo AA, Milić N, Cicala C, Santini A, Capasso R (2018) Milk thistle (Silybum marianum): a concise overview on its chemistry, pharmacological, and nutraceutical uses in liver diseases. Phytother Res 32:2202–2213
Adamu T, Mun B-G, Lee S-U, Hussain A, Yun B-W (2018) Exogenously applied nitric oxide enhances salt tolerance in rice (Oryza sativa L.) at seedling stage. Agronomy 8:276
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Aghajanlou F, Mirdavoudi H, Shojaee M, Mac Sweeney E, Mastinu A, Moradi P (2021) Rangeland management and ecological adaptation analysis model for Astragalus curvirostris Boiss. Horticulturae 7(4):67
Ahmad P, Abdel Latef AA, Hashem A, Abd-Allah EF, Gucel S, Tran L-SP (2016) Nitric oxide mitigates salt stress by regulating levels of osmolytes and antioxidant enzymes in chickpea. Front Plant Sci 7:347
Ahmad P, Abass Ahanger M, Nasser Alyemeni M, Wijaya L, Alam P, Ashraf M (2018) Mitigation of sodium chloride toxicity in Solanum lycopersicum L. by supplementation of jasmonic acid and nitric oxide. J Plant Interact 13:64–72
Akram NA, Iqbal M, Muhammad A, Ashraf M, Al-Qurainy F, Shafiq S (2017) Aminolevulinic acid and nitric oxide regulate oxidative defense and secondary metabolisms in canola (Brassica napus L.) under drought stress. Protoplasma 255:163–174
Alexieva V, Sergiev I, Mapelli S, Karanov E (2001) The effect of drought and ultraviolet radiation on growth and stress markers in pea and wheat. Plant, Cell Environ 24:1337–1344
Anjum MA (2011) Effect of exogenously applied spermidine on growth and physiology of citrus rootstock Troyer citrange under saline conditions. Turk J Agric for 35(1):43–53
Arasimowicz-Jelonek M, Floryszak-Wieczorek J, Kubiś J (2009) Involvement of nitric oxide in water stress-induced responses of cucumber roots. Plant Sci 177:682–690
Arnon DI (1949) Copper enzymes in isolated chloroplasts: polyphenoloxidase in Beta Vulgaris. Plant Physiol 24:1–15
Azizi I, Esmaielpour B, Fatemi H (2021) Exogenous nitric oxide on morphological, biochemical and antioxidant enzyme activity on savory (Satureja Hortensis L) plants under cadmium stress. J Saudi Soc Agric Sci 20(6):417–423
Banerjee P, Prasad B (2020) Determination of concentration of total sodium and potassium in surface and ground water using a flame photometer. Appl Water Sci 10(5):1–7
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207
Batista PF, Costa AC, Müller C, Silva-Filho RdO, Barbosa da Silva F, Merchant A, Mendes GC, Nascimento KJT (2018) Nitric oxide mitigates the effect of water deficit in Crambe abyssinica. Plant Physiol Biochem 129:310–322
Ben Rejeb K, Abdelly C, Savoure A (2014) How reactive oxygen species and proline face stress together. Plant Physiol Biochem 80:278–284
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Chance B, Maehly AC (1955) [136] Assay of catalases and peroxidases. 2: 764–775
da Silva CJ, Batista Fontes EP, Modolo LV (2017) Salinity-induced accumulation of endogenous H2S and NO is associated with modulation of the antioxidant and redox defense systems in Nicotiana tabacum L. cv. Havana Plant Sci 256:148–159
dos Santos TB, Ribas AF, de Souza SGH, Budzinski IGF, Domingues DS (2022) Physiological responses to drought, salinity, and heat stress in plants: a review. Stresses 2(1):113–135
Du Z, Bramlage WJ (2002) Modified thiobarbituric acid assay for measuring lipid oxidation in sugar-rich plant tissue extracts. J Agric Food Chem 40:1566–1570
Du X, Zhang C, Guo W, Jin W, Liang Z, Yan X, Guo Z, Liu Y, Yang D (2015) Nitric oxide plays a central role in water stress-induced tanshinone production in Salvia miltiorrhiza hairy roots. Molecules 20:7574–7585
El Moukhtari A, Cabassa-Hourton C, Farissi M, Savouré A (2020) How does proline treatment promote salt stress tolerance during crop plant development? Front Plant Sci 11:1127
Fan Q-J, Liu J-H (2011) Nitric oxide is involved in dehydration/drought tolerance in Poncirus trifoliata seedlings through regulation of antioxidant systems and stomatal response. Plant Cell Rep 31:145–154
Farouk S, Arafa SA (2018) Mitigation of salinity stress in canola plants by sodium nitroprusside application. Span J Agric Res 16:e0802
Fricke W, Akhiyarova G, Veselov D, Kudoyarova G (2004) Rapid and tissue-specific changes in ABA and in growth rate response to salinity in barley leaves. J Exp Bot 55:1115–1123
Fu Z-W, Wang Y-L, Lu Y-T, Yuan T-T (2016) Nitric oxide is involved in stomatal development by modulating the expression of stomatal regulator genes in Arabidopsis. Plant Sci 252:282–289
GalmÉS J, OchogavÍA JM, Gago J, RoldÁN EJ, Cifre J, Conesa MÀ (2013) Leaf responses to drought stress in Mediterranean accessions of Solanum lycopersicum: anatomical adaptations in relation to gas exchange parameters. Plant, Cell Environ 36:920–935
Garcia-Mata C, Lamattina L (2007) Abscisic acid (ABA) inhibits light-induced stomatal opening through calcium- and nitric oxide-mediated signaling pathways. Nitric Oxide 17:143–151
Ghadakchiasl A, Mozafari AA, Ghaderi N (2017) Mitigation by sodium nitroprusside of the effects of salinity on the morpho-physiological and biochemical characteristics of Rubus idaeus under in vitro conditions. Physiol Mol Biol Plants 23(1):73–83
Ghassemi-Golezani K, Lotfi R (2015) The impact of salicylic acid and silicon on chlorophyll a fluorescence in mung bean under salt stress. Russ J Plant Physiol 62:611–616
Ghavami N, Ramin AA (2008) Grain yield and active substances of milk thistle as affected by soil salinity. Commun Soil Sci Plant Anal 39(17–18):2608–2618. https://doi.org/10.1080/00103620802358672
Gupta P, Srivastava S, Seth CS (2016) 24-Epibrassinolide and sodium nitroprusside alleviate the salinity stress in Brassica juncea L. cv. Varuna through cross talk among proline, nitrogen metabolism and abscisic acid. Plant Soil 411:483–498
Hayat S, Yadav S, Wani AS, Irfan M, Ahmad A (2011) Nitric oxide effects on photosynthetic rate, growth, and antioxidant activity in tomato. Int J Veg Sci 17:333–348
Idrees M, Naeem M, Khan MN, Aftab T, Khan MMA, Moinuddin (2011) Alleviation of salt stress in lemongrass by salicylic acid. Protoplasma 249:709–720
Kalaji HM, Jajoo A, Oukarroum A, Brestic M, Zivcak M, Samborska IA, Cetner MD, Łukasik I, Goltsev V, Ladle RJ (2016) Chlorophyll a fluorescence as a tool to monitor physiological status of plants under abiotic stress conditions. Acta Physiol Plant 38:102
Karkanis A, Bilalis D, Efthimiadou A (2011) Cultivation of milk thistle (Silybum marianum L. Gaertn.), a medicinal weed. Ind Crops Prod 34:825–830
Karthik S, Pavan G, Krishnan V, Sathish S, Manickavasagam M (2019) Sodium nitroprusside enhances regeneration and alleviates salinity stress in soybean [Glycine max (L) Merrill]. Biocatal Agric Biotechnol 19:101173
Kaya C, Akram NA, Ashraf M, Sonmez O (2018) Exogenous application of humic acid mitigates salinity stress in maize (Zea mays L.) plants by improving some key physico-biochemical attributes. Cereal Res Commun 46:67–78
Kelly G, Egbaria A, Khamaisi B, Lugassi N, Attia Z, Moshelion M, Granot D (2019) Guard-cell hexokinase increases water-use efficiency under normal and drought conditions. Front Plant Sci 10:1499
Keshavarz Afshar R, Chaichi MR, Rezaei K, Asareh MH, Karimi M, Hashemi M (2015) Irrigation regime and organic fertilizers influence on oil content and fatty acid composition of milk thistle seeds. Agron J 107:187–194
Khan MN, Siddiqui MH, Mohammad F, Naeem M (2012) Interactive role of nitric oxide and calcium chloride in enhancing tolerance to salt stress. Nitric Oxide 27:210–218
Kumar A, Memo M, Mastinu A (2020) Plant behaviour: an evolutionary response to the environment? Plant Biol 22:961–970
Leshem YAY, Haramaty E (1996) The characterization and contrasting effects of the nitric oxide free radical in vegetative stress and senescence of Pisum sativum Linn. foliage. J Plant Physiol 148:258–263
Li Q-Y, Niu H-B, Yin J, Wang M-B, Shao H-B, Deng D-Z, Chen X-X, Ren J-P, Li Y-C (2008) Protective role of exogenous nitric oxide against oxidative-stress induced by salt stress in barley (Hordeum vulgare). Colloids Surf, B 65:220–225
Liao Q, Gu S, Kang S, Du T, Tong L, Wood JD, Ding R (2022) Mild water and salt stress improve water use efficiency by decreasing stomatal conductance via osmotic adjustment in field maize. Sci Total Environ 805:150364
Lichtenthaler HK (1987) Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods Enzymol 148:350–382
Liu J, Shi DC (2010) Photosynthesis, chlorophyll fluorescence, inorganic ion and organic acid accumulations of sunflower in responses to salt and salt-alkaline mixed stress. Photosynthetica 48:127–134
Liu L, Wang B (2021) Protection of halophytes and their uses for cultivation of saline-alkali soil in China. Biology 10(5):353. https://doi.org/10.3390/biology10050353
Liu JH, Nada K, Honda C, Kitashiba H, Wen XP, Pang XM, Moriguchi T (2006) Polyamine biosynthesis of apple callus under salt stress: importance of the arginine decarboxylase pathway in stress response. J Exp Bot 57(11):2589–2599
Liu S, Dong Y, Xu L, Kong J (2013) Effects of foliar applications of nitric oxide and salicylic acid on salt-induced changes in photosynthesis and antioxidative metabolism of cotton seedlings. Plant Growth Regul 73:67–78
Liu W, Zhang Y, Yuan X, Xuan Y, Gao Y, Yan Y (2016) Exogenous salicylic acid improves salinity tolerance of Nitraria tangutorum. Russ J Plant Physiol 63:132–142
Lutts S (1996) NaCl-induced senescence in leaves of rice (Oryza sativa L.) cultivars differing in salinity resistance. Ann Bot 78:389–398
Ma Y, Yao Y, Wang Q, Gu Z, Wang P, Han Y, Yang R (2021) Mechanism of nitric oxide enhancing NaCl tolerance of barley seedlings based on physiol-biochemical analysis and LC-MS metabolomics. Environ Exp Bot 189:104533
Machado S, Paulsen GM (2001) Combined effects of drought and high temperature on water relations of wheat and sorghum. Plant Soil 233:179–187
Mahdavi A, Moradi P, Mastinu A (2020) Variation in terpene profiles of Thymus vulgaris in water deficit stress response. Molecules 25(5):1091
Malone SR, Mayeux HS, Johnson HB, Polley HW (1993) Stomatal density and aperture length in four plant species grown across a subambient CO2 gradient. Am J Bot 80:1413
Mioto PT, Mercier H (2013) Abscisic acid and nitric oxide signaling in two different portions of detached leaves of Guzmania monostachia with CAM up-regulated by drought. J Plant Physiol 170:996–1002
Mohammadi MH, Khataar M, Shekari F (2017) Effect of soil salinity on the wheat and bean root respiration rate at low matric suctions. Paddy Water Environ, 15:639–648
Mohammadreza S, Amin B, Forogh A, Hossin M, Sorayya S (2012) Response of Brassica napus L. grains to the interactive effect of salinity and salicylic acid. J Stress Physiol Biochem 8:159–166
Mohasseli V, Sadeghi S (2019) Exogenously applied sodium nitroprusside improves physiological attributes and essential oil yield of two drought susceptible and resistant specie of thymus under reduced irrigation. Ind Crops Prod 130:130–136
Molassiotis A, Tanou G, Diamantidis G (2014) NO says more than ‘YES’ to salt tolerance. Plant Signal Behav 5:209–212
Morant-Manceau A, Pradier E, Tremblin G (2004) Osmotic adjustment, gas exchanges and chlorophyll fluorescence of a hexaploid triticale and its parental species under salt stress. J Plant Physiol 161:25–33
Mostofa MG, Fujita M, Tran L-SP (2015) Nitric oxide mediates hydrogen peroxide- and salicylic acid-induced salt tolerance in rice (Oryza sativa L.) seedlings. Plant Growth Regul 77:265–277
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Annu Rev Plant Biol 59:651–681
Nabi RBS, Tayade R, Hussain A, Kulkarni KP, Imran QM, Mun B-G, Yun B-W (2019) Nitric oxide regulates plant responses to drought, salinity, and heavy metal stress. Environ Exp Bot 161:120–133
Naheed R, Aslam H, Kanwal H, Farhat F, Abo Gamar MI, Al-Mushhin AAM, Jabborova D, Ansari MJ, Shaheen S, Aqeel M, Noman A, Hessini K (2021) Growth attributes, biochemical modulations, antioxidant enzymatic metabolism and yield in Brassica napus varieties for salinity tolerance. Saudi J Biol Sci 28(10):5469–5479
Nakano Y, Asada K (1981) Hydrogen-peroxide is scavenged by ascorbate-specific peroxidase in spinach-chloroplasts. Plant Cell Physiol 22:867–880
Nasiri-Savadkoohi S (2017) Protective role of exogenous nitric oxide against zinc toxicity in Plantago major L. Appl Ecol Environ Res 15:511–524
Ning P, Wang J, Zhou Y, Gao L, Wang J, Gong C (2016) Adaptional evolution of trichome in Caragana korshinskii to natural drought stress on the Loess Plateau, China. Ecol Evol 6:3786–3795
Rad SV, Valadabadi SAR, Pouryousef M, Saifzadeh S, Zakrin HR, Mastinu A (2020) Quantitative and qualitative evaluation of Sorghum bicolor L. under intercropping with legumes and different weed control methods. Horticulturae 6(4):78
Radoglou KM, Jarvis PG (1990) Effects of CO2 enrichment on four poplar clones: II—leaf surface properties. Ann Bot 65:627–632
Ragaey MM, Sadak MS, Dawood MFA, Mousa NHS, Hanafy RS, Latef AAHA (2022) Role of signaling molecules sodium nitroprusside and arginine in alleviating salt-induced oxidative stress in wheat. Plants 11(14):1786
Ramadan AA, Abd Elhamid EM, Sadak MS (2019) Comparative study for the effect of arginine and sodium nitroprusside on sunflower plants grown under salinity stress conditions. Bull Natl Res Centre 43:118
Rasheed R, Ashraf MA, Ali S, Iqbal M, Zafar S (2022) Plant metabolism adjustment in exogenously applied NO under stress. Nitric oxide in plant biology. Academic Press, Cambridge, pp 261–296
Sami F, Faizan M, Faraz A, Siddiqui H, Yusuf M, Hayat S (2018) Nitric oxide-mediated integrative alterations in plant metabolism to confer abiotic stress tolerance, NO crosstalk with phytohormones and NO-mediated post translational modifications in modulating diverse plant stress. Nitric Oxide 73:22–38
Santisree P, Bhatnagar-Mathur P, Sharma KK (2015) NO to drought-multifunctional role of nitric oxide in plant drought: do we have all the answers? Plant Sci 239:44–55
Shi J, Gao L, Zuo J, Wang Q, Wang Q, Fan L (2016) Exogenous sodium nitroprusside treatment of broccoli florets extends shelf life, enhances antioxidant enzyme activity, and inhibits chlorophyll-degradation. Postharv Biol Technol 116:98–104
Singh H, Singh NB, Singh A, Hussain I, Yadav V (2017) Oxidative stress induced by lead in Vigna radiata L. seedling attenuated by exogenous nitric oxide. Trop Plant Res 4:225–234
Singh-Tomar R, Mathur S, Allakhverdiev SI, Jajoo A (2012) Changes in PS II heterogeneity in response to osmotic and ionic stress in wheat leaves (Triticum aestivum). J Bioenerg Biomembr 44:411–419
Sousa LF, Menezes-Silva PE, Lourenço LL, Galmés J, Guimarães AC, Silva AF, Reis Lima AP, Henning LMM, Costa AC, Silva FG, Farnese FdS (2019) Improving water use efficiency by changing hydraulic and stomatal characteristics in soybean exposed to drought: the involvement of nitric oxide. Physiol Plant 168:576–589
Sun L, Li Y, Miao W, Piao T, Hao Y, Hao F-S (2017) NADK2 positively modulates abscisic acid-induced stomatal closure by affecting accumulation of H2O2, Ca2+ and nitric oxide in Arabidopsis guard cells. Plant Sci 262:81–90
Surender Reddy P, Jogeswar G, Rasineni GK, Maheswari M, Reddy AR, Varshney RK, Kavi Kishor PB (2015) Proline over-accumulation alleviates salt stress and protects photosynthetic and antioxidant enzyme activities in transgenic sorghum [Sorghum bicolor (L.) Moench]. Plant Physiol Biochem 94:104–113
Tang AC (2002) Photosynthetic oxygen evolution at low water potential in leaf discs lacking an epidermis. Ann Bot 89:861–870
Wei L, Derrien B, Gautier A, Houille-Vernes L, Boulouis A, Saint-Marcoux D, Malnoë A, Rappaport F, de Vitry C, Vallon O, Choquet Y, Wollman F-A (2014) Nitric oxide–triggered remodeling of chloroplast bioenergetics and thylakoid proteins upon nitrogen starvation in Chlamydomonas reinhardtii. Plant Cell 26:353–372
Wendehenne D, Pugin A, Klessig DF, Durner J (2001) Nitric oxide: comparative synthesis and signaling in animal and plant cells. Trends Plant Sci 6(4):177–183
Wodala B, Deák Z, Vass I, Erdei L, Altorjay I, Horváth F (2008) In vivo target sites of nitric oxide in photosynthetic electron transport as studied by chlorophyll fluorescence in pea leaves. Plant Physiol 146:1920–1927
Xiong J, Zhang L, Fu G, Yang Y, Zhu C, Tao L (2011) Drought-induced proline accumulation is uninvolved with increased nitric oxide, which alleviates drought stress by decreasing transpiration in rice. J Plant Res 125:155–164
Xu L, Baldocchi DD, Tang J (2004) How soil moisture, rain pulses, and growth alter the response of ecosystem respiration to temperature. Glob Biogeochem Cycles 18:GB4002
Xu MJ, Dong JF, Zhu MY (2005) Nitric oxide mediates the fungal elicitor-induced hypericin production of Hypericum perforatum cell suspension cultures through a jasmonic-acid-dependent signal pathway. Plant Physiol 139(2):991–998. https://doi.org/10.1104/pp.105.066407
Yang Z, Li J-L, Liu L-N, Xie Q, Sui N (2020) Photosynthetic regulation under salt stress and salt-tolerance mechanism of sweet sorghum. Front Plant Sci 10:1722. https://doi.org/10.3389/fpls.2019.01722
Yasir TA, Khan A, Skalicky M, Wasaya A, Rehmani MIA, Sarwar N, Mubeen K, Aziz M, Hassan MM, Hassan FAS, Iqbal MA, Brestic M, Islam MS, Danish S, El Sabagh A (2021) Exogenous sodium nitroprusside mitigates salt stress in lentil (Lens culinaris Medik.) by affecting the growth, yield, and biochemical properties. Molecules 26:2576
Yousefvand P, Sohrabi Y, Heidari G, Weisany W, Mastinu A (2022) Salicylic acid stimulates defense systems in Allium hirtifolium grown under water deficit stress. Molecules 27(10):3083
Yu Y, Zhao C, Zhao Z, Yu B, Zhou T (2015) Soil respiration and the contribution of root respiration of cotton (Gossypium hirsutum L.) in arid region. Acta Ecol Sin 35:17–21
Zahra N, Wahid A, Hafeez MB, et al. (2021) Plant growth promoters mediated quality and yield attributes of milk thistle (Silybum marianum L.) ecotypes under salinity stress. Sci Rep 11:23200. https://doi.org/10.1038/s41598-021-02435-4
Zangani E, Zehtab-Salmasi S, Andalibi B, Zamani A-A (2018) Protective effects of nitric oxide on photosynthetic stability and performance of Silybum marianum under water deficit conditions. Agron J 110:555–564
Zangani E, Afsahi K, Shekari F, Mac Sweeney E, Mastinu A (2021a) Nitrogen and phosphorus addition to soil improves seed yield, foliar stomatal conductance, and the photosynthetic response of rapeseed (Brassica napus L.). Agriculture 11:483
Zangani E, Zehtab-Salmasi S, Andalibi B, Zamani AA, Hashemi M (2021b) Exogenous nitric oxide improved production and content of flavonolignans in milk thistle seeds under water deficit system. Acta Physiol Plant 43:87
Zhang Y, Wang L, Liu Y, Zhang Q, Wei Q, Zhang W (2006) Nitric oxide enhances salt tolerance in maize seedlings through increasing activities of proton-pump and Na+/H+ antiport in the tonoplast. Planta 224:545–555
Zhang LP, Mehta SK, Liu ZP, Yang ZM (2008) Copper-induced proline synthesis is associated with nitric oxide generation in Chlamydomonas reinhardtii. Plant Cell Physiol 49:411–419
Zhao GQ, Ma BL, Ren CZ (2007) Growth, gas exchange, chlorophyll fluorescence, and ion content of naked oat in response to salinity. Crop Sci 47:123–131
Zhou B, Guo Z, Xing J, Huang B (2005) Nitric oxide is involved in abscisic acid-induced antioxidant activities in Stylosanthes guianensis. J Exp Bot 56:3223–3228
Acknowledgements
The authors would like to thank all the technicians who assisted us in performing the experiments.
Funding
Open access funding provided by Università degli Studi di Brescia within the CRUI-CARE Agreement. This work was supported by the University of Zanjan and University of Brescia, Italy.
Author information
Authors and Affiliations
Contributions
EZ conceived the study; EZ, AA, and FS performed biochemical and morphological analyses and helped interpret the results. AA, FS, BA, and KA provided plant care, performed the physiological analyses, and helped interpret the results. EZ, AA, and AM interpreted the results, wrote the original draft of the manuscript, and performed statistical analysis, review, and editing of the manuscript.
Corresponding authors
Additional information
Handling Editor: Parvaiz Ahmad.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zangani, E., Ansari, A., Shekari, F. et al. Alleviating the Injuries of NaCl Exposure on Respiratory Activities, Leaf Stomatal and Antioxidant Defense of Silybum marianum L. Seedlings by Exogenous Nitric Oxide. J Plant Growth Regul 42, 7731–7748 (2023). https://doi.org/10.1007/s00344-023-11045-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-023-11045-5