Abstract
Purpose
To evaluate the feasibility and safety of transcatheter arterial embolization (TAE) with the use of HepaSphere microspheres for hypervascular tumors.
Materials and methods
This was a prospective multicenter open label clinical trial involving six institutions in Japan. TAE was performed with the HepaSphere microspheres for hypervascular tumors in various locations. The endpoint of the study was the feasibility and safety of the procedure. The feasibility and safety were evaluated according to technical success and adverse events, respectively. Adverse events that were related to TAE were evaluated by using the Common Terminology Criteria for Adverse Events, version 4.0.
Results
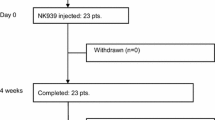
Twenty-four patients were enrolled. The technical success rate was 100 % (24/24). Twenty-two (92 %) patients developed a total of 50 symptomatic adverse events, including 30 grade 1 events, 17 grade 2 events, and 3 grade 3 events. The most frequent adverse event was fever with an incidence of 63 %, followed by abdominal pain (25 %).
Conclusion
TAE for hypervascular tumors with the HepaSphere microspheres was feasible and rarely caused major adverse events.
Trial registration: This trial was registered in JAPIC Clinical Trials Information (JapicCTI-111534).
Similar content being viewed by others
References
Nakamura H, Hashimoto T, Oi H, Sawada S. Transcatheter oily chemoembolization of hepatocellular carcinoma. Radiology. 1989;170:783–6.
Uchida H, Ohishi H, Matsuo N, et al. Transcatheter hepatic segmental arterial embolization using lipiodol mixed with an anticancer drug and Gelfoam particles for hepatocellular carcinoma. Cardiovasc Intervent Radiol. 1990;13(3):140–5.
Hodaka ATY, Hidehiko K. A Case of non-functioning islet cell carcinoma with multiple liver metastases–cemplete responce to transcatheter arterial ernbolization. Jpn J Gastroenterol Surg. 1992;25:3002–6.
Yamada R, Sato M, Kawabata M, Nakatsuka H, Nakamura K, Takashima S. Hepatic artery embolization in 120 patients with unresectable hepatoma. Radiology. 1983;148(2):397–401.
Takayasu K, Arii S, Ikai I, et al. Prospective cohort study of transarterial chemoembolization for unresectable hepatocellular carcinoma in 8510 patients. Gastroenterology. 2006;131(2):461–9.
Katsumori T, Kasahara T. The size of gelatin sponge particles: differences with preparation method. Cardiovasc Intervent Radiol. 2006;29(6):1077–83.
Yamada R, Sawada S, Uchida H, et al. Clinical study of porous gelatin sphere (YM 670) in transcatheter arterial embolization. Gan to kagaku ryoho. 2005;32(10):1431–6.
Osuga K, Anai H, Takahashi M, et al. Porous gelatin particles for hepatic arterial embolization; investigation of the size distribution and fragmentation before and after microcatheter passage. Gan to kagaku ryoho. 2009;36:437–42.
Laurent A. Microspheres and nonspherical particles for embolization. Tech Vasc Interv Radiol. 2007;10(4):248–56.
Lewis AL, Adams C, Busby W, et al. Comparative in vitro evaluation of microspherical embolisation agents. J Mater Sci Mater Med. 2006;17(12):1193–204.
Laurent A, Beaujeux R, Wassef M, Rufenacht D, Boschetti E, Merland JJ. Trisacryl gelatin microspheres for therapeutic embolization, I: development and in vitro evaluation. AJNR Am J Neuroradiol. 1996;17(3):533–40.
Beaujeux R, Laurent A, Wassef M, et al. Trisacryl gelatin microspheres for therapeutic embolization, II: preliminary clinical evaluation in tumors and arteriovenous malformations. AJNR Am J Neuroradiol. 1996;17(3):541–8.
de Luis E, Bilbao JI, de Ciercoles JA, de Martinez-Cuesta A, Martino Rodriguez A, Lozano MD. In vivo evaluation of a new embolic spherical particle (HepaSphere) in a kidney animal model. Cardiovasc Intervent Radiol. 2008;31(2):367–76.
Hori S, Okada A, Sakamoto K. A new embolic material: superabsorbent polymer microsphere and its embolic effects. Jpn J Interv Radiol. 1996;11:375–81.
Lee KH, Liapi EA, Cornell C, et al. Doxorubicin-loaded QuadraSphere microspheres: plasma pharmacokinetics and intratumoral drug concentration in an animal model of liver cancer. Cardiovasc Intervent Radiol. 2010;33(3):576–82.
Lewis AL, Gonzalez MV, Lloyd AW, et al. DC bead: in vitro characterization of a drug-delivery device for transarterial chemoembolization. J Vasc Interv Radiol. 2006;17:335–42.
Osuga K, Hori S, Hiraishi K, et al. Bland embolization of hepatocellular carcinoma using superabsorbent polymer microspheres. Cardiovasc Intervent Radiol. 2008;31(6):1108–16.
Grosso M, Vignali C, Quaretti P, et al. Transarterial chemoembolization for hepatocellular carcinoma with drug-eluting microspheres: preliminary results from an Italian multicentre study. Cardiovasc Intervent Radiol. 2008;31(6):1141–9.
Jiaqi Y, Hori S, Minamitani K, et al. A new embolic material: super absorbent polymer (SAP) microsphere and its embolic effects. Nippon Igaku Hoshasen Gakkai Zasshi. 1996;56(1):19–24.
Chen AP, Setser A, Anadkat MJ, et al. Grading dermatologic adverse events of cancer treatments: the common terminology criteria for adverse Events Version 4.0. J Am Acad Dermatol. 2012;67(5):1025–39.
Khankan AA, Osuga K, Hori S, Morii E, Murakami T, Nakamura H. Embolic effects of superabsorbent polymer microspheres in rabbit renal model: comparison with tris-acryl gelatin microspheres and polyvinyl alcohol. Radiat Med. 2004;22(6):384–90.
Seki A, Hori S, Sueyoshi S, Hori A, Kono M, Murata S, et al. Transcatheter arterial embolization with spherical embolic agent for pulmonary metastases from renal cell carcinoma. Cardiovasc Intervent Radiol. 2013;36:1527–35.
Seki A, Hori S, Kobayashi K, Narumiya S. Transcatheter arterial chemoembolization with epirubicin-loaded superabsorbent polymer microspheres for 135 hepatocellular carcinoma patients: single-center experience. Cardiovasc Intervent Radiol. 2011;34(3):557–65.
Huppert P, Wenzel T, Wietholtz H. Transcatheter arterial chemoembolization (TACE) of colorectal cancer liver metastases by irinotecan-eluting microspheres in a salvage patient population. Cardiovasc Intervent Radiol. 2014;37(1):154–64.
Jarzabek M, Jargiello T, Wolski A, Poluha P, Szczerbo-Trojanowska M. Drug-eluting microspheres transarterial chemoembolization (DEM TACE) in patients with liver metastases. Pilot study. Pol J Radiol. 2011;76(3):26–32.
Acknowledgments
This study was funded by Nippon Kayaku Co., Ltd. All study sites and institutions received funding from Nippon Kayaku for clinical trial execution. We thank Hiraku Yodono, Tadashi Kimura, and Takafumi Ichida for evaluation of the technical success as members of an independent assessment committee.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Yoshihiro Nambu is an employee of Nippon Kayaku. Yasuaki Arai and Shinichi Hori received consultation and lecture fees from Nippon Kayaku. Takao Hiraki will receive honoraria for drafting this manuscript from Nippon Kayaku after publication of the article. Jun Koizumi, Yasuo Sakurai, and Hiromitsu Kumada declare that they have no conflicts of interest.
Ethical statement
All procedures performed in studies involving human participants were in accordance with “good clinical practice” (GCP), all applicable subject privacy requirements, and the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
About this article
Cite this article
Hiraki, T., Koizumi, J., Arai, Y. et al. Transcatheter arterial embolization of hypervascular tumors with HepaSphere: prospective multicenter open label clinical trial of microspheres in Japan. Jpn J Radiol 33, 479–486 (2015). https://doi.org/10.1007/s11604-015-0448-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11604-015-0448-8