Abstract
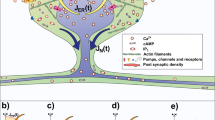
Dendritic spines are small, mushroom-like protrusions from the arbor of a neuron in the central nervous system. Interdependent changes in the morphology, biochemistry, and activity of spines have been associated with learning and memory. Moreover, post-mortem cortices from patients with Alzheimer’s or Parkinson’s disease exhibit biochemical and physical alterations within their dendritic arbors and a reduction in the number of dendritic spines. For over a decade, experimentalists have observed perforations in postsynaptic densities on dendritic spines after induction of long-term potentiation, a sustained enhancement of response to a brief electrical or chemical stimulus, associated with learning and memory. In more recent work, some suggest that activity-dependent intraspine calcium may regulate the surface area of the spine head, and reorganization of postsynaptic densities on the surface.
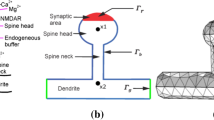
In this paper, we develop a model of a dendritic spine with the ability to partition its transmission and receptor zones, as well as the entire spine head. Simulations are initially performed with fixed parameters for morphology to study electrical properties and identify parameters that increase efficacy of the synaptic connection. Equations are then introduced to incorporate calcium as a second messenger in regulating continuous changes in morphology. In the model, activity affects compartmental calcium, which regulates spine head morphology. Conversely, spine head morphology affects the level of local activity, whether the spines are modeled with passive membrane properties, or excitable membrane using Hodgkin–Huxley kinetics. Results indicate that merely separating the postsynaptic receptors on the surface of the spine may add to the diversity of circuitry, but does not change the efficacy of the synapse. However, when the surface area of the spine is a dynamic variable, efficacy of the synapse may vary continuously over time.
Similar content being viewed by others
References
Almeida, C.G., Tampellini, D., Takahashi, R.H., Greengard, P., Lin, M.T., Snyder, E.M., Gouras, G.K., 2005. Beta-amyloid accumulation in APP mutant neurons reduces PSD-95 and GluR1 in synapses. Neurbiol. Dis. 20(2), 187–198.
Aradi, I., Holmes, W.R., 1999. Role of multiple calcium and calcium-dependent conductances in regulation of hippocampal dentate granule cell excitability. J. Comput. Neurosci. 6, 215–235.
Araya, R., Nikolenko, V., Eisenthal, K.B., Yuste, R., 2007. Sodium channels amplify spine potentials. Proc. Natl. Acad. Sci. USA 104(30), 12347–12352.
Baer, S.M., Rinzel, J., 1991. Propagation of dendritic spikes mediated by excitable spines: a continuum theory. J. Neurophysiol. 65, 874–890.
Barinaga, M., 2000. Synapses call the shots (review article). Science 290, 736–738.
Brauer, F., Nohel, J.A., 1969. Qualitative Theory of Ordinary Differential Equation. Benjamin, New York.
Deller, T., Mundel, P., Frotscher, M., 2000. Potential role of synaptopodin in spine motility by coupling actin to the spine apparatus. Hippocampus 10(5), 569–581.
Edwards, F.A., 1995. LTP—a structural model to explain the inconsistencies. Trends Neurosci. 18(6), 250–255.
Fiala, J.C., Allwardt, B., Harris, K.M., 2002. Dendritic spines do not split during hippocampal LTP or maturation. Nat. Neurosci. 5(4), 297–298.
Geinisman, Y., deToledo-Morrell, L., Morrell, F., Heller, R.E., Rossi, M., Parshall, R.F., 1993. Structural synaptic correlate of long-term potentiation: Formation of axospinous synapses with multiple, completely partitioned transmission zones. Hippocampus 3(4), 435–446.
Geinisman, Y., deToledo-Morrell, L., Morrell, F., Persina, I.S., Beatty, M.A., 1996. Synapse restructuring associated with the maintenance phase of hippocampal long-term potentiation. J. Comp. Neurol. 368(6), 413–423.
Gurkiewicz, M., Korngreen, A., 2007. A numerical approach to ion channel modelling using whole-cell voltage-clamp recordings and a genetic algorithm. PLoS Comput. Biol. 3(8), 169.
Halpain, S., Hipolito, A., Saffer, L., 1998. Regulation of F-actin stability in dendritic spines by glutamate receptors and calcineurin. J. Neurosci. 18, 9835–9844.
Harris, K.M., 1999a. Calcium from internal stores modifies dendritic spine shape. Proc. Natl. Acad. Sci. 96, 12213–12215.
Harris, K.M., 1999b. Structure, development, and plasticity of dendritic spines. Curr. Opin. Neurobiol. 9, 343–348.
Hausser, M., Spruston, N., Stuart, G.L., 2000. Diversity and dynamics of dendritic signaling. Science 290, 739–744.
Hodgkin, A., Huxley, A., 1952. A quantitative description of membrane current and its application to conduction and excitation in nerve. J. Physiol. (Lond.) 117, 500–544.
Holmes, W.R., Levy, W.B., 1997. Quantifying the role of inhibition in associative long-term potentiation in dentate granule cells with computational models. J. Neurophysiol. 78, 103–116.
Johnston, D., Miao-Sin Wu, S., 1995. Foundations of Cellular Neurophysiology. MIT Press, Cambridge.
Korkortian, E., Segal, M., 1999. Release of calcium from stores alters the morphology of dendritic spines in cultured hippocampal neurons. Proc. Natl. Acad. Sci. 96, 12068–12072.
Luscher, C., Nicoll, R.A., Malenka, R.C., Muller, D., 2000. Synaptic plasticity and dynamic modulation of the postsynaptic membrane (review article). Nat. Neurosci. 3(6), 545–550.
Mahadomrongkul, V., Huerta, P.T., Shirao, T., Aoki, C., 2005. Stability of the distribution of spines containing drebrin A in the sensory cortex lay I of mice expressing mutated APP and PS1 genes. Brain Res. 164(1–2), 66–74.
Matus, A., 2000. Actin based plasticity in dendritic spines. Science 290, 754–758.
Miller, J.P., Rall, W., Rinzel, J., 1985. Synaptic amplification by active membrane in dendritic spines. Brain Res. 325, 325–330.
Mogilner, A., Verzi, D.W., 2003. A simple 1-D physical model for the crawling nematode sperm cell. J. Stat. Phys. 110, 1169–1189.
Neuhoff, H., Roeper, J., Schweizer, M., 1999. Activity-dependent formation of perforated synapses in cultured hippocampal neurons. Eur. J. Neurol. 11, 4241–4250.
Rall, W., 1953. Electrotonic theory for spherical neurone. Proc. Univ. Otago Med. Sch. 31, 14–15.
Rose, C.R., Konnerth, A., 2001. NMDA receptor-mediated Na+ signals in spines and dendrites. J. Neurosci. 21(12), 4207–4214.
Rose, C.R., Kovalchuk, Y., Eilers, J., Konnerth, A., 1999. Two-photon Na+ imaging in spines and fine dendrites of central neurons. Eur. J. Physiol. 439(1–2), 201–207.
Rusakov, D.A., Stewart, M.G., Korogod, S.M., 1996. Branching of active dendritic spines as a mechanism for controlling synaptic efficacy. Neuroscience 75(1), 315–323.
Schmid, G., Hannggi, P., 2007. Intrinsic coherence resonance in excitable membrane patches. Math. Biosci. 207(2), 235–245.
Segev, I., London, M., 2000. Untangling dendrites with quantitative models. Science 290, 744–749.
Segev, I., Rall, W., 1988. Computational study of an excitable dendritic spine. J. Neurophysiol. 60, 499–523.
Segev, I., Rall, W., 1998. Excitable dendrites and spines: Earlier theoretical insights elucidate recent direct observations. Trends Neurosci. 21, 453–460.
Sorra, K.E., Fiala, J.C., Harris, K.M., 1999. Critical assessment of the involvement of perforations, spinules, and spine branching in hippocampal synapse formation. J. Comp. Neurol. 398, 225–240.
Stephens, B., Mueller, A.J., Shering, A.F., Hood, S.H., Taggart, P., Arbuthnott, G.W., Bell, J.E., Kilford, L., Kingsbury, A.E., Daniel, S.E., Ingham, C.A., 2005. Evidence of a breakdown of corticostriatal connections in Parkinson’s disease. Neuroscience 132(3), 741–754.
Timofeeva, Y., Lord, G.J., Coombes, S., 2006. Spatio-temporal filtering properties of a dendritic cable with active spines: A modeling study in the spike-diffuse-spike framework. J. Comput. Neurosci. 21, 293–306.
Verzi, D.W., 2004. Modeling activity-dependent synapse restructuring. B. Math. Biol. 66, 745–762.
Verzi, D.W., Baer, S.M., 2005. Calcium-mediated spine stem restructuring. J. Math. Comput. Model. 42, 151–165.
Verzi, D.W., Baer, S.M., Rheuben, M.B., 2005. Impact of time-dependent changes in spine density and spine shape on the input–output properties of a dendritic branch: A computational study. J. Neurophysiol. 93, 2073–2089.
Waltman, P., 1986. A Second Course in Elementary Differential Equations. Academic, Orlando.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Verzi, D.W., Noris, O.Y. A Compartmental Model for Activity-Dependent Dendritic Spine Branching. Bull. Math. Biol. 71, 1048–1072 (2009). https://doi.org/10.1007/s11538-009-9393-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11538-009-9393-y