Abstract
Purpose
The objective of the present study was to investigate the interactive effects of nitrogen (N) addition, temperature, and moisture on soil microbial respiration, microbial biomass, and metabolic quotient (qCO2) at different decomposition stages of different tree leaf litters.
Materials and methods
A laboratory incubation experiment with and without litter addition was conducted for 80 days at two temperatures (15 and 25 °C), two wetting intensities (35 and 50 % water-filled porosity space (WFPS)) and two doses of N addition (0 and 4.5 g N m−2, as NH4NO3). The tree leaf litters included three types of broadleaf litters, a needle litter, and a mixed litter of them. Soil microbial respiration, microbial biomass, and qCO2 along with other soil properties were measured at two decomposition stages of tree leaf litters.
Results and discussion
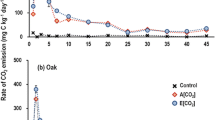
The increase in soil cumulative carbon dioxide (CO2) flux and microbial biomass during the incubation depended on types of tree leaf litters, N addition, and hydrothermal conditions. Soil microbial biomass carbon (C) and N and qCO2 were significantly greater in all litter-amended than in non-amended soils. However, the difference in the qCO2 became smaller during the late period of incubation, especially at 25 °C. The interactive effect of temperature with soil moisture and N addition was significant for affecting the cumulative litter-derived CO2-C flux at the early and late stages of litter decomposition. Furthermore, the interactive effect of soil moisture and N addition was significant for affecting the cumulative CO2 flux at the late stage of litter decomposition but not early in the experiment.
Conclusions
This present study indicated that the effects of addition of N and hydrothermal conditions on soil microbial respiration, qCO2, and concentrations of labile C and N depended on types of tree leaf litters and the development of litter decomposition. The results highlight the importance of N availability and hydrothermal conditions in interactively regulating soil microbial respiration and microbial C utilization during litter decomposition under forest ecosystems.






Similar content being viewed by others
References
Aerts R (1997) Climate, leaf litter chemistry and leaf litter decomposition in terrestrial ecosystems: a triangular relationship. Oikos 79:439–449
Bertrand I, Delfosse O, Mary B (2007) Carbon and nitrogen mineralization in acidic, limed and calcareous agricultural soils: apparent and actual effects. Soil Biol Biochem 39:276–288
Boberg JB, Finlay RD, Stenlid J, Näsholm T, Lindahl BD (2008) Glucose and ammonium additions affect needle decomposition and carbon allocation by the litter degrading fungus Mycena epipterygia. Soil Biol Biochem 40:995–999
Boberg JB, Näsholm T, Finlay RD, Stenlid J, Lindahl BD (2011) Nitrogen availability affects saprotrophic basidiomycetes decomposing pine needles in a long term laboratory study. Fungal Ecol 4:408–416
Bowden RD, Davidson E, Savage K, Arabia C, Steudler P (2004) Chronic nitrogen additions reduce total soil respiration and microbial respiration in temperate forest soils at the Harvard forest. For Ecol Manag 196:43–56
Boyle D (1998) Nutritional factors limiting the growth of Lentinula edodes and other white-rot fungi in wood. Soil Biol Biochem 30:817–823
Carreiro MM, Sinsabaugh RL, Repert DA, Parkhurst DF (2000) Microbial enzyme shifts explain litter decay responses to simulated nitrogen deposition. Ecology 81(9):2359–2365
Compton JE, Watrud LS, Porteous LA, DeGrood S (2004) Response of soil microbial biomass and community composition to chronic nitrogen additions at Harvard forest. For Ecol Manag 196:143–158
Conde E, Cardenas M, Ponce-Mendoza A, Luna-Guido ML, Cruz-Mondragon C, Dendooven L (2005) The impacts of inorganic nitrogen application on mineralization of C-14-labelled maize and glucose, and on priming effect in saline alkaline soil. Soil Biol Biochem 37:681–691
Cornwell WK, Cornelissen JHC, Amatangelo K, Dorrepaa E, Eviner VT, Godoy O, Hobbie SE, Hoorens B, Kurokawa H, Perez-Harguindeguy N, Quested HM, Santiago LS, Wardle DA, Wright IJ, Aerts R, Allison SD, vanBodegom P, Brovkin V, Chatain A, Callaghan TV, Dıaz S, Garnier E, Gurvich DE, Kazakou E, Klein JA, Read J, Reich PB, Soudzilovskaia NA, Vaieretti MV, Westoby M (2008) Plant species traits are the predominant control on litter decomposition rates within biomes worldwide. Ecol Lett 11:1065–1071
de Graaff MA, van Groenigen KJ, Six J, Hungate B, van Kessel C (2006) Interactions between plant growth and soil nutrient cycling under elevated CO2: a meta-analysis. Glob Chang Biol 12:2077–2091
DeForest JL, Zak DR, Pregitzer KS, Burton AJ (2004) Atmospheric nitrate deposition, microbial community composition, and enzyme activity in northern hardwood forests. Soil Sci Soc Am J 68:132–138
Ding WX, Yu HY, Cai ZC, Han FX, Xu ZH (2010) Responses of soil respiration to N fertilization in a loamy soil under maize cultivation. Geoderma 155:381–389
Dixon RK, Brown S, Houghton RA, Solomon AM, Trexler MC, Wisniewski J (1994) Carbon pools and flux of global forest ecosystems. Science 263:185–190
Ekblad A, Nordgren A (2002) Is growth of soil microorganisms in boreal forests limited by carbon or nitrogen availability? Plant Soil 242:115–122
Fang H, Mo JM, Peng SL, Li Z, Wang H (2007) Cumulative effects of nitrogen additions on litter decomposition in three tropical forests in southern China. Plant Soil 297(1):233–242
Fernandez I, Mahieu N, Cadisch G (2003) Carbon isotope fractionation during decomposition of plant materials of different quality. Glob Biogeochem Cycles 17(3):1075. doi:10.1029/GB001834
Fierer N, Craine JM, McLauchlan K, Schimel JP (2005) Litter quality and the temperature sensitivity of decomposition. Ecology 86(2):320–326
Fog K (1988) The effect of added nitrogen on the rate of decomposition of organic-matter. Biol Rev 63(3):433–462
Franklin O, Hogberg P, Ekblad A, Agren GI (2003) Pine forest floor carbon accumulation in response to N and PK additions: bomb C-14 modelling and respiration studies. Ecosystems 6:644–658
Franzluebbers AJ (1999) Microbial activity in response to water-filled pore space of variably eroded southern Piedmont soils. Appl Soil Ecol 11:91–101
Frey SD, Knorr M, Parrent JL, Simpson RT (2004) Chronic nitrogen enrichment affects the structure and function of the soil microbial community in temperate hardwood and pine forests. For Ecol Manag 196:159–171
Guo RH, Zheng JQ, Han SJ, Zhang JH, Li MH (2013) Carbon and nitrogen turnover in response to warming and nitrogen addition during early stages of forest litter decomposition—an incubation experiment. J Soils Sediments 13:312–324
Harris D, Voroney RP, Paul EA (1997) Measurement of microbial biomass N:C by chloroform fumigation-incubation. Can J Soil Sci 77:507–514
Hart SC, Stark JM (1997) Nitrogen limitation of the microbial biomass in an old-growth forest soil. Ecoscience 4:91–98
He YT, Xu XL, Kueffer C, Zhang XZ, Shi PL (2014) Leaf litter of a dominant cushion plant shifts nitrogen mineralization to immobilization at high but not low temperature in an alpine meadow. Plant Soil 383:415–426
Heal OW, Anderson JM, Swift MJ (1997) Plant litter quality and decomposition: an historical overview. In: Cadisch G, Giller KE (eds) Driven by nature: plant litter quality and decomposition. CAB International, Wallingford, pp 3–30
Hobbie SE (1996) Temperature and plant species control over litter decomposition in Alaskan Tundra. Ecol Monogr 66(4):503–522
Hobbie SE (2000) Interactions between litter lignin and soil nitrogen availability during leaf litter decomposition in a Hawaiian Montane forest. Ecosystems 3(5):484–494
Janssens IA, Dieleman W, Luyssaert S, Subke JA, Reichstein M, Ceulemans R, Ciais P, Dolman AJ, Grace J, Matteucci G (2010) Reduction of forest soil respiration in response to nitrogen deposition. Nat Geosci 3:315–322
Jenkinson DS (1988) The determination of microbial biomass carbon and nitrogen in soil. In: Wilson JR (ed) Advances in nitrogen cycling in agricultural ecosystems. CAB International, Wallingford, pp 368–386
Jenkinson DS, Brooks PC, Powlson DS (2004) Measuring soil microbial biomass. Soil Biol Biochem 36:5–7
King HGC, Heath G (1967) Chemical analysis of small samples of leaf material and relationship between disappearance and composition of leaves. Pedobiologia 7:192–197
Knorr M, Frey SD, Curtis PS (2005) Nitrogen additions and litter decomposition: a meta-analysis. Ecology 86:3252–3257
Lee K, Jose S (2003) Soil respiration, fine root production, and microbial biomass in cottonwood and loblolly pine plantations along a nitrogen fertilization gradient. For Eco Manag 185:263–273
Li XF, Han SJ, Zhang Y (2007) Foliar decomposition in a broadleaf-mixed Korean pine (Pinus koraiensis Sieb. Et Zucc) plantation forest: the impact of initial litter quality and the decomposition of three kinds of organic matter fraction on mass loss and nutrient release rates. Plant Soil 295:151–167
Li XF, Hu YL, Han SJ, Liu Y, Zhang Y (2010) Litterfall and litter chemistry change over time in an old-growth temperate forest, northeastern China. Ann For Sci 67:206
Lipson DA, Schmidt SK, Monson RK (2000) Carbon availability and temperature control the post-snowmelt decline in alpine soil microbial biomass. Soil Biol Biochem 32:441–448
Magill AH, Aber JD (1998) Long-term effects of experimental nitrogen additions on foliar litter decay and humus formation in forest ecosystems. Plant Soil 203(2):301–311
McAndrew DW, Malhi SS (1992) Long-term N fertilization of a Solonetzic soil: effects on chemical and biological properties. Soil Biol Biochem 24:619–623
Melillo JM, Aber JD, Muratore JF (1982) Nitrogen and lignin control of hardwood leaf litter decomposition dynamics. Ecology 1982:621–626
Neely CL, Beare MH, Hargrove WL, Coleman DC (1991) Relationships between fungal and bacterial substrate-induced respiration, biomass and plant residue decomposition. Soil Biol Biochem 23:947–954
Neff JC, Townsend AR, Gleixner G, Lehman SJ, Turnbull J, Bowman WD (2002) Variable effects of nitrogen additions on the stability and turnover of soil carbon. Nature 419:915–917
Ni K, Ding WX, Cai ZC, Wang YF, Zhang XL, Zhou BK (2012) Soil carbon dioxide emission from intensively cultivated black soil in Northeast China: nitrogen fertilization effect. J Soils Sediments 12:1007–1018
Paul EA, Clark FE (1996) Soil microbiology and biochemistry. Academic, San Diego, pp 109–115
Rasmussen C, Southard RJ, Horwath WR (2008) Litter type and soil minerals control temperate forest soil carbon response to climate change. Glob Chang Biol 14:2064–2080
Ryan MG, Melillo JM, Ricca A (1990) A comparison methods for determining proximate carbon fractionation of forest litter. Can J For Res 20:166–171
Salah YMS, Scholes MC (2011) Effect of temperature and litter quality on decomposition rate of Pinus patula needle litter. Procedia Environ Sci 6:180–193
Sall SN, Masse D, Bernhard-Reversat F, Guisse A, Chotte JL (2003) Microbial activities during the early stage of laboratory decomposition of tropical leaf litters: the effect of interactions between litter quality and exogenous inorganic nitrogen. Biol Fertil Soils 39:103–111
Scowcroft PG, Turner DR, Vitousek PM (2000) Decomposition of Metrosideros polymorpha leaf litter along elevational gradients in Hawaii. Glob Chang Biol 6(1):73–85
Štursová M, Žifčáková L, Leigh MB, Burgess R, Baldrian P (2012) Cellulose utilization in forest litter and soil: identification of bacterial and fungal decomposers. FEMS Microbiol Ecol 80:735–746
Tan X, Chang SX (2007) Soil compaction and forest litter amendment affect carbon and net nitrogen mineralization in a boreal forest soil. Soil Tillage Res 93(1):77–86
Teklay T, Nordgren A, Nyberg G, Malmer A (2007) Carbon mineralization of leaves from four Ethiopian agroforestry species under laboratory and field conditions. Appl Soil Ecol 35(1):193–202
Thirukkumaran CM, Parkinson D (2000) Microbial respiration, biomass, metabolic quotient and litter decomposition in a lodgepole pine forest floor amended with nitrogen and phosphorous fertilizers. Soil Biol Biochem 32:59–66
Treseder KK (2008) Nitrogen additions and microbial biomass: a meta-analysis of ecosystem studies. Ecol Lett 11:1111–1120
Vestgarden LS (2001) Carbon and nitrogen turnover in early stage Scots pine (Pinus sylvestris L.) needle litter decomposition: effects of internal and external nitrogen. Soil Biol Biochem 33:465–474
Waldrop MP, Zak DR (2006) Response of oxidative enzyme activities to nitrogen deposition affects soil concentrations of dissolved organic carbon. Ecosystems 9:921–933
Wallander H, Nilsson LO, Hagerberg D, Rosengren U (2003) Direct estimates of C:N ratios of ectomycorrhizal mycelia collected from Norway spruce forest soils. Soil Biol Biochem 35:997–999
Wetterstedt JÅM, Persson T, ÅGren GI (2010) Temperature sensitivity and substrate quality in soil organic matter decomposition: results of an incubation study with three substrates. Glob Chang Biol 16(6):1806–1819
Wu J, Joergensen RG, Pommerening B, Chaussod R, Brookes PC (1990) Measurement of soil microbial biomass C by fumigation extraction—an automated procedure. Soil Biol Biochem 22:1167–1169
Xu XK, Han L, Wang YS, Inubushi K (2007) Influence of vegetation types and soil properties on microbial biomass carbon and metabolic quotients in temperate volcanic and tropical forest soils. Soil Sci Plant Nutr 53:430–440
Zak DR, Holmes WE, Burton AJ, Pregitzer KS, Talhelm AF (2008) Simulated atmospheric NO3 deposition increases soil organic matter by slowing decomposition. Ecol Appl 18:2016–2027
Zhang Q, Zak JC (1998) Effects of water and nitrogen amendment on soil microbial biomass and fine root production in a semi-arid environment in West Texas. Soil Biol Biochem 30:39–45
Acknowledgments
This research was funded by the National Natural Science Foundation of China (41175133, 41275166, 41321064, and 41575154). The authors thank three anonymous reviewers for their constructive comments and assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Zhihong Xu
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 89.1 kb)
Rights and permissions
About this article
Cite this article
Xu, X., Yin, L., Duan, C. et al. Effect of N addition, moisture, and temperature on soil microbial respiration and microbial biomass in forest soil at different stages of litter decomposition. J Soils Sediments 16, 1421–1439 (2016). https://doi.org/10.1007/s11368-015-1331-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-015-1331-z