Abstract
Purpose
A 7-month field experiment was conducted to investigate the polycyclic aromatic hydrocarbon (PAH) remediation potential of two plant species and changes in counts of soil PAH-degrading bacteria and microbial activity.
Materials and methods
Alfalfa and tall fescue were grown in monoculture and intercropped for 7 months in contaminated field soil. Soil and plant samples were analyzed for PAHs. Plant biomass, densities of PAH-degradation soil bacteria, soil microbial biomass C and N, enzyme activities, and the physiological profile of the soil microbial community were determined.
Results and discussion
Average removal percentage of total PAHs in intercropping (30.5%) was significantly higher than in monoculture (19.9%) or unplanted soil (−0.6%). About 7.5% of 3-ring, 12.3% of 4-ring, and 17.2% of 5(+6)-ring PAHs were removed from the soil by alfalfa, with corresponding values of 25.1%, 10.4%, and 30.1% for tall fescue. Intercropping significantly enhanced the remediation efficiency. About 18.9% of 3-ring, 30.9% of 4-ring, and 33.4% of 5(+6)-ring PAHs were removed by the intercropping system. Higher counts of soil culturable PAH-degrading bacteria and elevated microbial biomass and enzyme activities were found after intercropping. Soil from intercropping showed significantly higher (p < 0.05) average well-color development obtained by the BIOLOG Ecoplate assay and Shannon–Weaver index compared with monoculture.
Conclusions
Cropping promoted the dissipation of soil PAHs. Tall fescue gave greater removal of soil PAHs than alfalfa, and intercropping was more effective than monoculture. Intercropping of alfalfa and tall fescue may be a promising in situ bioremediation strategy for PAH-contaminated soils.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Polycyclic aromatic hydrocarbons (PAHs) are of considerable concern because they are potentially toxic to humans and are persistent contaminants in the environment (Wilcke 2007). Phytoremediation, the use of plants to remediate contaminated soil, has been described as a promising approach to remediate soils contaminated with persistent organic pollutants such as PAHs (Chen et al. 2003; Huang et al. 2004; Phillips et al. 2009). During the last few decades, numerous plant species including alfalfa and tall fescue have been found to be promising candidates for phytoremediation of PAHs (Liu et al. 2004; Parrish et al. 2004; Balcom and Crowley 2009). Parrish et al. (2004) reported 24% dissipation of total PAHs in composted soil in the presence of tall fescue. Greenhouse experiments have demonstrated enhanced removal of PAHs when alfalfa was planted compared with unplanted controls (Liu et al. 2004). These results were obtained using single plant species and little is known about how plant species and cropping patterns affect the processes by which phytoremediation removes PAHs on a field scale (Meng et al. 2011; Wei and Pan 2010). More information is, therefore, required on the in situ phytoremediation potential of intercropped species such as alfalfa and tall fescue.
The ultimate goals of any remediation approach must be to remove the contaminants from the soil and to restore the capacity of the soil to function according to its potential (Epelde et al. 2009). Soil microorganisms are very sensitive to any ecosystem function shifts because their activity and diversity are rapidly altered by perturbation (Margesin et al. 2000; Andreoni et al. 2004; Joner et al. 2004). Microbiological parameters such as microbial biomass, enzyme activities, and the diversity of soil microbial communities may serve as important indices of the impact of pollution on soil health (Labud et al. 2007; Epelde et al. 2008; Teng et al. 2010; Tu et al. 2011). The aims of the present work were, therefore, to investigate the in situ phytoremediation potential of intercropped alfalfa and tall fescue and to examine associated changes in microbial activities in a PAH-contaminated soil.
2 Materials and methods
2.1 Chemicals
A chromatographic grade standard mixture consisting of the 16 PAHs prioritized by the US EPA (naphthalene, acenaphthylene, acenaphthene, fluorene, phenanthrene, anthracene, fluoranthene, pyrene, benzo[a]anthracene, chrysene, benzo[b]fluoranthene, benzo[k]fluoranthene, benzo[a]pyrene, dibenz[a,h]anthracene, benzo[ghi]perylene, and indeno[1,2,3-cd]pyrene) was purchased from AccuStandard, Inc. (New Haven, CT). All the other chemicals used were of analytical or chromatographic grade.
2.2 Site description and soil preparation
The study was conducted on contaminated agricultural land adjacent to an iron and steel foundry in Jiangsu province, southeast China. Uncontrolled emissions of gases and dust from the factory have resulted in PAH pollution over a 40-year period, and there are no other known pollution sources in the area. Initial total concentrations of 16 PAH congeners in the soil ranged from 747 to 810 μg kg−1. Selected soil properties were as follows: pH 6.40; organic carbon, 24.9 g kg−1; total N, 1.2 g kg−1; total P, 0.6 g kg−1; and total K, 15.2 g kg−1. Soil pH (soil/H2O ratio of 1:2) was measured using a pH meter with a glass electrode. Soil organic C and total N were determined by dichromate oxidation and Kjeldahl digestion and distillation, respectively. Soil total P was extracted by digestion with persulphate and determined by spectrophotometry. Soil total K was extracted by digestion with concentrated HF-HClO4 and measured by flame emission or atomic absorption spectrophotometry.
2.3 Plants
Seeds of Medicago sativa and Festuca arundinacea were purchased from Nanjing Agricultural University Seed Company. After surface sterilization in a 10% (v/v) solution of hydrogen peroxide for 10 min and rinsing with sterile distilled water, seeds were germinated and seedlings grown for 7 days in moist perlite before transfer to the plots. Seedlings of uniform size were selected and then transplanted to the designated plots.
2.4 Experimental design and sample collection
The contaminated field site was divided into 12 plots, each 1.6 × 2.2 m. There were four treatments set up in a fully randomized layout with each treatment in triplicate. The four treatments were (1) alfalfa monoculture (A), (2) tall fescue monoculture (T), (3) alfalfa intercropped with tall fescue (A/T), and (4) unplanted control soil with neither alfalfa nor tall fescue. In the intercropping treatment, four rows of alfalfa were intercropped with four rows of tall fescue, and the single cropping plots consisted of eight rows of one plant species. The same inter-row distance of 0.30 m was used in all the planted treatments in accordance with local agricultural practice. Seedlings in each row were thinned 7 days after transplanting to leave 50 seedlings of alfalfa or tall fescue in monoculture plots and 25 seedlings of alfalfa and 25 of tall fescue in intercropping plots. Throughout the growing season the plants were monitored daily and watered as necessary and once a week weeds were removed by hand from the plots.
At the end of the experiment soil samples were collected by taking five random soil samples to a depth of 15 cm from each plot using a corer auger. The samples were combined to give one composite sample per plot. All field samples were taken at time zero and collected for analysis after 7 months of plant growth. The soil was sieved to 2 mm and divided into several subsamples, which were stored differently depending on the analysis to be carried out. Portions were stored at 4°C for microbial assays that were performed within 1 week. In addition, 15 plants were harvested from each plot and carefully rinsed with distilled water to remove any remaining soil and dust particles. The plant samples and the remainder of the soil samples were freeze-dried and stored at 4°C.
2.5 Extraction and analysis of soil and plant PAHs
PAHs in bulk soil samples were extracted using Soxhlet extraction. In brief, 5 g of freeze-dried sample with filter paper was placed in a porous cellulose thimble (25 × 70 mm) and placed in a Soxhlet extractor. The extractor was then fitted to a 100 ml round bottom flask containing 60 ml dichloromethane, and the extraction was performed for 24 h. All the extracts in the round bottom flasks were dried by rotary evaporation. The residues were dissolved in 2 ml of cyclohexane and 0.5 ml of the solution was transferred and purified with a silica gel column (8 × 220 mm) and washed with a mixture of hexane and dichloromethane (1:1). The first 1 ml of eluate was discarded because it contained nonpolar saturated hydrocarbons and had lower retention than PAHs on silica gel. The second 2-ml aliquot of eluate was collected, dried by sparging with N2, and then re-dissolved in 1 ml acetonitrile for high-performance liquid chromatography (HPLC).
Plant samples were ground and homogenized and subsamples were extracted by ultrasonication for 1 h in sufficient 1:1 (v/v) solution of acetone and hexane. The solvent was then decanted, collected, and replenished. The sample was sonicated for 1 h, and the solvent was decanted, collected, and replenished again. This process was repeated three times. The solvent fractions were combined and passed through an anhydrous Na2SO4 column with elution of 1:1 (v/v) acetone and hexane. The solvents were then evaporated and taken up in 2 ml hexane, followed by filtration through 2 g of silica gel in a column and eluted with 11 ml of a 1:1 (v/v) mixture hexane and dichloromethane. The samples were then evaporated and taken up in acetonitrile with a final volume of 2 ml for HPLC determination (Reilley et al. 1996; Kipopoulou et al. 1999).
Determination of 16 EPA PAHs was carried out according to the method of Ni et al. (2008). Briefly, analysis was conducted on a Shimadzu Class-VP HPLC system (Shimadzu, Japan) with a fluorescence detector (RF-10AXL). A reversed phase column C18 (VP-ODS, 150 × 4.6 mm I.D. and particle size, 5 mm), using a mobile phase of water and acetonitrile mixture (1:9, v/v) at a constant solvent flow rate of 0.5 ml min−1, was used to separate the 16 PAHs. The excitation and emission wavelengths for individual PAHs were set separately.
An external standard mixture was used for quantification of the 16 PAHs. The detection limit of the HPLC method for the 16 PAHs was in the range 0.12 to 1.57 μg kg−1. Method blanks (solvent) and spiked blanks (soil spiked with standards of EPA610 PAH mixture, LA 96245, Supelco, Bellefonte, PA) were extracted and analyzed by the methods described above. The recoveries and the relative standard deviations of this method for 16 PAHs were in the ranges of 74% to 110% and 0.53% to 3.57%, respectively. Results of blanks extracted under the same conditions were below detection limit and sample results are presented without recovery ratio correction.
The percentage of PAH removal (%) was calculated by the following formula:
where Ms was the concentration of PAHs in each treatment and Mi was the initial PAH concentration present in the soil.
2.6 Microbial activity
2.6.1 Counts of soil PAH-degrading bacteria
PAH-degrading soil bacteria were counted using a miniaturized most probable number (MPN) method in 96-well microplates with five replicates per dilution (Wrenn and Venosa 1996). Briefly, phenanthrene, anthracene, fluorene, and dibenzothiophene were added as the sole carbon sources to support the proliferation of aromatics-degrading bacteria. The wells were inoculated with serially diluted samples, and the microplates were incubated at room temperature for 3 weeks. Wells turning yellow or brown owing to the accumulation of partial oxidation products of aromatic substrates were treated as positive. Published MPN tables were used to determine the MPN values.
2.6.2 Soil microbial biomass C and N
Soil microbial biomass C and N were determined by the fumigation–extraction method (Brookes et al. 1985; Vance et al. 1987). Chloroform fumigation was carried out with ethanol-free CHCl3 for 24 h at 25°C in the dark. The CHCl3 was removed and 10 g soil samples extracted by shaking with 50 ml 0.5 mol L−1 K2SO4 for 30 min on a rotary shaker. The suspensions were then filtered through Whatman no. 42 filter paper. Triplicate subsamples of unfumigated control soils were stored at 4°C during fumigation and were extracted in the same way and at the same time as the fumigated samples. Organic C was measured with an automated TOC Analyzer (Shimadzu, TOC-500, Japan). Biomass C (Bc) was calculated as \( {\text{Bc}} = {2}.{22} \times {\text{Ec}} \), where Ec = [(organic C extracted from fumigated soil) − (organic C extracted from non-fumigated soil)]. The factor 2.22 is a proportionality constant, accounting for the observation that about 45% of biomass C is extracted after fumigation. Soil microbial biomass N was estimated from the relationship: \( {\text{biomass}}\;{\text{N}} = {\text{EN}}/{\text{kEN}} \), where EN is total N extracted from fumigated soil minus total N extracted from non-fumigated soil and kEN = 0.54 (Brookes et al. 1985).
2.6.3 Soil enzyme activities
Soil dehydrogenase activity (DHA) was assessed by a modification of the method described by Singh and Singh (2005). Weighed 5-g subsamples of soil were placed in 50 ml polypropylene centrifuge tubes and mixed with 5 ml 0.5% 1,3,5-triphenyltetrazolium chloride (TTC) solution. Tubes were incubated for 6 h at 30°C in the dark. After incubation, triphenylformazan (TPF) formed by reduction of TTC was extracted with three batches of 100 ml methanol. Tubes were shaken in an orbital shaker at 300 rpm for 1 h, centrifuged (2,000 rpm, 5 min), and the supernatant was filtered with filter paper. Blanks without the addition of TTC were carried out in the same manner. The concentration of TPF was determined by spectrophotometry at 485 nm and the results are expressed as g TPF g−1 soil.
Urease activity was assessed by a modification of the method described by Gianfreda et al. (1994). Soil samples (20 g) were placed in 100 ml volumetric flasks, 2 ml toluene was added to the soil in each flask, and the contents were allowed to stand for approximately 15 min until the toluene had completely penetrated the soil. Then 20 ml potassium-citric acid buffer (pH 7.6) and 10 ml 10% urea solution were added. The flasks were shaken and then incubated at 37°C for 6 h. A control in which 10-ml distilled water was substituted for the urea was run simultaneously for each soil sample. For convenience, the incubation time of 3 h used in the original method was increased to 6 h without significant influence on the rate of urea hydrolysis. After incubation the contents of the flasks were diluted to 100 ml with distilled water and the toluene formed an immiscible layer above the graduation mark. The flasks were shaken well and the contents filtered through a Whatman no. 5 filter paper. The clear filtrate varied from colorless through yellow to brown depending on the amount of soluble organic matter present. The ammonia released by hydrolysis of urea was determined in the filtrate by the colorimetric indophenol blue method. The influence of colored extracts, and any ammonia initially present on the exchange complex and extracted from the soil, was accounted for in the measurement of the control. The concentration of NH4–N was determined by spectrophotometry at 578 nm and the results are expressed as μg NH4–N released g−1 dry soil h−1 incubation.
2.6.4 Physiological profiles of the soil microbial community
Soil microbial community level physiological profiles were performed as described by Yao et al. (2003). Briefly, 10 g of fresh soil was added to 100 ml of distilled water in a 250-ml flask and shaken for 10 min. Tenfold serial dilutions were made and the 10−3 dilution was used to inoculate BIOLOG® ECOplates (BIOLOG, Hayward, CA). The plates were incubated at 25°C and color development in each well was recorded as optical density (OD) at 590 nm with a plate reader at regular 12-h intervals. Microbial activity in each microplate, expressed as average well-color development (AWCD), was determined as follows:
where OD i is the optical density value from each well. The Shannon–Weaver index (H) was calculated using an OD of 0.25 as the threshold for positive response (Garland 1996). The Shannon–Weaver index was calculated using the Eq. H = −Σp i LnP i , where p i is the ratio of the activity on each substrate (OD i ) to the sum of activities on all substrates (∑OD i ).
2.7 Statistical analysis
All statistical analysis was carried out using the SPSS 14.0 for Windows software package. Data were analyzed by one-way analysis of variance, and mean values were compared by least significant difference (LSD) at the 5% level. Principal component analysis (PCA) was conducted to further distinguish the extent of differentiation of different treatments with regard to soil bacterial community carbon utilization profiles.
3 Results
3.1 Shoot biomass and accumulation of PAHs in plants
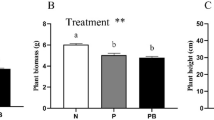
Plant aboveground dry matter yields in the different treatments are presented in Table 1. Tall fescue in monoculture (T) produced the highest yield (p < 0.05). The average dry matter yields of alfalfa in monoculture, tall fescue in monoculture and the two species in mixture were 1.82, 5.55, and 3.27 t ha−1, respectively.
Alfalfa and tall fescue in a mixture (A/T) exhibited the highest PAH concentrations in plants followed by tall fescue in monoculture (T), and alfalfa in monoculture (A), between which there was no significant difference (p > 0.05) (see Table 1). The average PAH concentrations in plants of treatments A, T, and A/T were 190, 231, 270/284 μg kg−1 dry biomass, respectively. The average PAH concentrations in plants of the A/T treatment were significantly higher than those of both A and T treatments (p < 0.05), but no significant difference was observed in PAH concentrations between the A and T treatments (p > 0.05).
3.2 Dissipation of PAHs in soil
Concentrations of total PAHs in soil under the different treatments after 7 months of phytoremediation are presented in Fig. 1. By the end of the experiment soil total PAH concentrations in intercropping (A/T) plots, sole cropping (A and T) plots and unplanted control plots averaged 546 ± 18.2, 644 ± 18.9, 632 ± 14.5, 817 ± 27.7 μg kg−1 dry soil, respectively. Compared with the initial soil PAH concentrations, significant differences (p < 0.05) were observed in treatments A/T, A, and T in which 30.4%, 14.4%, and 24.0% of total PAHs were degraded (Table 2).
Dissipation of total PAHs and 3-, 4-, and 5(+6)-ring PAHs in soil before and after 7 months of phytoremediation. PAH concentration values are means±standard deviations of triplicate determinations. Mean values with the same letter are not significantly different among treatments by LSD test at the 5% level. A alfalfa monoculture, T tall fescue monoculture, A/T alfalfa and tall fescue in mixture, CK unplanted control, Before before 7 months of phytoremediation, After after 7 months of phytoremediation
Figure 1 also shows the concentrations of 3-, 4-, and 5(+6)-ring PAHs in soil under the different treatments. In treatment A/T, the residual levels of 3-, 4-, and 5(+6)-ring PAHs were 74 ± 4.7, 249 ± 7.1, and 222 ± 8.7 μg kg−1 dry soil, representing 18.9%, 30.9%, and 33.4% removal from the initial soil values. Compared with the control, significant degradation (p < 0.05) was observed in 3-, 4-, and 5(+6)-ring PAHs in the A/T intercropping plots. In treatment A, the residual levels of 3-, 4-, and 5(+6)-ring PAHs were 84 ± 7.7, 302 ± 10.7, and 258 ± 10.0 μg kg−1 dry soil, with 7.5%, 12.3%, and 17.2% degradation in the A sole cropping plots. In treatment T the residual levels of 3-, 4-, and 5(+6)-ring PAHs were 81 ± 5.7, 308 ± 12.4, and 229 ± 10.8 μg kg−1 dry soil, representing 25.1%, 10.4%, and 30.1% dissipation in the T sole cropping plots.
3.3 Percent mass removal and phytoextraction of PAHs from soil
The effect of alfalfa and tall fescue cropping on percent mass removal and phytoextraction of PAHs from the soil are shown in Table 2. The volume of soil in each hectare of 10,000 m2 × 15 cm multiplied by the mean soil density of 1.28 g cm−3 gives a soil mass of 1,920,000 kg, and this value can be used to calculate the absolute quantity of soil PAHs in the plots to a depth of 15 cm (see Table 2). The percent removal rates of target PAHs by the A, tall T, and A/T were 14.5%, 24.4%, and 30.5%, respectively. In the control soil, −0.6% of the initial PAH level was observed. For the A/T and alfalfa and tall fescue in monoculture (A and T), percentage of PAHs phytoextracted from soil were 0.20%, 0.04%, and 0.32%, respectively.
3.4 Soil PAH-degrading bacteria and microbial activities
Soil PAH-degrading bacterial counts after 7 months of phytoremediation are presented in Table 3. Compared with the control, significantly higher counts (p < 0.05) were observed in mixture (A/T) plots. Furthermore, bacterial counts in mixture (A/T) plots (\( 4.2\pm 0.6 \times {10^{3}}\:{\text{MPN}}\:{{\text{g}}^{{ - 1}}}\:{\text{dry}}\:{\text{soil}} \)) were 2.3 and 1.7 times higher than in A (\( 1.8\pm 0.1 \times {10^{3}}\:{\text{MPN}}\:{{\text{g}}^{{ - 1}}}\:{\text{dry}}\:{\text{soil}} \)) and T (\( 2.5\pm 0.3 \times {10^{3}}\:{\text{MPN}}\:{{\text{g}}^{{ - 1}}}\:{\text{dry}}\:{\text{soil}} \)) monoculture, respectively. However, there was no significant interaction between alfalfa and tall fescue.
After 7 months of phytoremediation all treatments (A, T, and A/T) showed higher microbial biomass C and N (p < 0.05) compared with the control (see Table 3). Soil microbial biomass C and N in mixture soil (A/T) were 172 ± 7.3 μg C g−1 dry soil and 30 ± 5.4 μg N g−1 dry soil, increases of 70% and 312% compared with control soil. Furthermore, both microbial biomass C and N in mixture soil (A/T) were significantly higher (p < 0.05) than in monoculture (A and T) soil.
Higher DHA were observed in the soil after 7 months of phytoremediation, with significant enhancement (p < 0.05) in both monoculture (A and T) and mixture (A/T) plots (82 ± 4.2, 81 ± 5.5, and 92 ± 7.8 μg TPF g−1 dry soil, respectively) (see Table 3). Soil DHA activities in monoculture (A and T) and A/T plots were 2.12-, 2.07- and 2.36-fold higher than those measured in the control. Both monocrop (A 144 ± 7.1and T 146 ± 4.8 μg NH4–N g−1 dry soil) and intercrop plots (186 ± 7.0 μg NH4–N g−1 dry soil) also showed significantly higher (p < 0.05) soil urease activities after 7 months of phytoremediation compared with the control (113 ± 9.3 μg NH4–N g−1 dry soil) (see Table 3). The urease activity of A/T intercropping plots was significantly higher than that in monoculture plots (A and T), but there was no significant difference (p > 0.05) between T and A monocultures. However, there was a significant interaction between alfalfa and tall fescue.
3.5 Physiological profiles of soil bacterial community
Variation in AWCD after 7 months of phytoremediation is shown in Fig. 2. Both the A/T and A plots showed much higher AWCD than the T and CK plots in soil bacterial community carbon utilization profiles at the end of the incubation, but there was no significant difference between the A/T and A plots at the point of the 156th hour (p > 0.05). Soil from A/T, A, and T plots showed significantly greater carbon utilization than that from the control plots (p < 0.05).
Variation in average well-color development (AWCD) for soil samples from the different treatments after 7 months of phytoremediation. Values are means±standard deviations of triplicate measurements. A alfalfa in monoculture, T tall fescue in monoculture, A/T alfalfa and tall fescue in mixture, CK unplanted control
H of soil microbial communities in the A/T, A, T, and CK plots after 7 months of phytoremediation were 3.28 ± 0.45, 3.29 ± 0.66, 3.19 ± 0.02, and 2.55 ± 0.49, respectively. The Shannon–Weaver index was higher in A/T plots and in A and T plots compared with the unplanted controls. The A/T and A plots showed significant differences (p < 0.05) in the Shannon–Weaver index compared with the T plots and control plots. There was no significant difference (p > 0.05) between the A/T plots and A plots.
PCA was also conducted to further distinguish the extent of differentiation of different treatments with regard to soil bacterial community carbon utilization profiles (Fig. 3). The first and second principal components (PC1 and PC2) explained 58.8% and 14.1% of the variance in the data. The A plots were closer to the T plots, and A, T, and A/T plots were differentiated from the control plots. The carbon sources significantly correlated with PC1 and PC2 (r > 0.70) under the different treatments are shown in Table 4. Substrates methyl pyruvate, Tween 40, liver sugar, d-mannitol, 2-dihydroxybenzoic acid, itaconic acid, d-malic acid, l-serine, l-threonine, glycyl-l-glutamic acid, β-methyl-d-glucuronide hydrate, i-erythritol, d-galacturonic acid, and l-arginine were intensively metabolized by soil microbial communities in A/T plots. Soil microbial communities from A plots mainly used liver sugar, α-d-lactose, N-acetyl-d-glucosamine, d-galacturonic acid, d-galactoic acid, d-malic acid, l-serine, glycyl-l-glutamic acid, γ-lactone, d(+)-cellobiose, itaconic acid, l-asparagine, and alfadex as their carbon substrates. As for T plots, the main carbon sources metabolized were β-methyl-d-glucuronide hydrate, glucose-1-phosphate, d-galacturonic acid, 2-dihydroxybenzoic acid, itaconic acid, d-malic acid, phenethylamine, putrescine, Tween 80, d-xylose, d-mannitol, and α-oxobutanoic acid.
4 Discussion
Phytoremediation is a viable choice for PAH remediation if sufficient time is allowed for plant establishment and contaminant degradation. In the process, the plants can be used to extract, detoxify, and/or sequester toxic pollutants from soil. Phytoremediation has now emerged as a promising strategy for in situ removal of numerous soil contaminants (Macek et al. 2000; Susarla et al. 2002; Pilon-Smits 2005; Åslund et al. 2007; Chen et al. 2003) although there are still few examples in commercial practice. The present study was conducted to investigate the capability of different plant species to promote the remediation of PAH-contaminated soil in field conditions. The major finding of the study was that the presence of plants significantly enhanced the dissipation of PAHs in the soil and planting alfalfa and tall fescue in mixture achieved the highest value. This suggests that planting alfalfa and tall fescue in mixture played an important role in the remediation of the PAH-contaminated soil. This result confirmed the findings from Denys (2006) who found a decrease in soil total PAHs of 25% in a mixture of herbaceous species grown in PAH-contaminated soil in the field. Although the present study shows an overall improvement in dissipation of total PAHs in intercropped treating, this is primarily due to a significantly enhanced dissipation of 4-ring PAHs in A/T plots compared with T plots. The discrepancy may have resulted from the use of different plant species and associated microbial groups in the rhizosphere exerting different effects on soil PAHs (Singleton et al. 2005, 2006).
Although plants have taken up certain quantities of PAHs, the absolute amount of PAHs stored in plant compartments makes little contribution to the removal of total soil PAHs. Calculations using maximum plant uptake in all plants indicated that phytoextraction removed less than 1% of the total PAH mass in the soil, which was consistent with Meng et al. (2011) and Gao and Zhu (2004) that plants take up less than 2% of total soil PAHs. This suggested that there must be some indirect phytoremediation strategies besides the direct extraction of soil PAHs by alfalfa and tall fescue. It has been reported that plants can also accelerate bioremediation by releasing secondary compounds like simple sugars, amino acids and flavonoids that stimulate the growth of specific microbial communities in the soil (Macek et al. 2000) or possibly induce enzyme systems of existing bacterial populations. In the present study, soil dehydrogenase, and urease were selected as indicators to determine the general microbial activities in the soil. Table 3 shows that the presence of alfalfa and tall fescue significantly increased the activities of both enzymes during the phytoremediation. Similar results were also described by Margesin et al. (2000). Moreover, both soil enzyme activities increased in the planting alfalfa and tall fescue in mixture than alfalfa and tall fescue in monoculture. Based on these observations, it could be concluded that planting alfalfa and tall fescue in mixture enhanced the degradation of soil PAHs by stimulating microbial activities in the soil.
Results from the examination of soil microbial biodiversity also show a significant enhancement on bacterial communities in the planting treatments. AWCD, Shannon–Weaver index and PCA of BIOLOG data distinguished clear differentiation between cropping treatments and unplanted control soil, which was also observed for carbon substrates that belonged to different microbial categories. Intercropping alfalfa with tall fescue may be a promising system for the phytoremediation of PAH-contaminated soil with at least partial restoration of the microbiological functioning of the contaminated soil.
5 Conclusions
Phytoremediation appears to have great potential for treatment of PAH-contaminated soils. Compared with monoculture, plants in mixture significantly enhanced the rate of PAH degradation in the soil, particularly high molecular weight PAHs such as 4- and 5(+6)-ring PAHs. Intercropping systems can increase soil PAH-degrading bacterial counts and microbial activities, suggesting that alfalfa and tall fescue growing together can restore the microbiological functioning of PAH-contaminated soil. Thus, alfalfa and tall fescue intercropping is a promising phytoremediation strategy for PAH-contaminated soil. Full elucidation of the potential applicability of this intercropping system will require further studies in a range of soil types and for different contaminants.
References
Andreoni V, Cavalca L, Rao MA, Nocerino G, Bernasconi S, Dell'Amico E, Colombo M, Gianfreda L (2004) Bacterial communities and enzyme activities of PAHs polluted soils. Chemosphere 57:401–412
Åslund MLW, Zeeb BA, Rutter A, Reimer KJ (2007) In situ phytoextraction of polychlorinated biphenyl—(PCB) contaminated soil. Sci Total Environ 374:1–12
Balcom IN, Crowley DE (2009) Pyrene effects on rhizoplane bacterial communities. Int J Phytoremediat 11:609–622
Brookes PC, Landman A, Pruden G, Jenkinson DS (1985) Chloroform fumigation and the release of soil nitrogen: a rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biol Biochem 17:837–842
Chen YC, Katherinebanks M, Paulschwab A (2003) Pyrene degradation in the rhizosphere of tall fescue (Festuca arundinacea) and switchgrass (Panicum virgatum L). Environ Sci Technol 37:5778–5782
Denys S (2006) In-situ phyroremediationof PAHs contaminated soils following a bioremediation treatment. Water Air Soil Pollut Focus 6:299–315
Epelde L, Becerril JM, Hernández-Allica J, Barrutia O, Garbisu C (2008) Functional diversity as indicator of the recovery of soil health derived from Thlaspi caerulescens growth and metal phytoextraction. Appl Soil Ecol 39:299–310
Epelde L, Mijangos I, Becerril JM, Garbisu C (2009) Soil microbial community as bioindicator of the recovery of soil functioning derived from metal phytoextraction with sorghum. Soil Biol Biochem 41:1788–1794
Gao YZ, Zhu LZ (2004) Plant uptake, accumulation and translocation of phenanthrene and pyrene in soils. Chemosphere 55:1169–1178
Garland JL (1996) Analytical approaches to the characterization of sample microbial communities using patterns of potential C source utilization. Soil Biol Biochem 28:213–221
Gianfreda L, Sannino F, Ortega N, Nannipieri P (1994) Activity of free and immobilized urease in soil: effect of pesticide. Soil Biol Biochem 26(6):777–784
Huang XD, El-Alawi Y, Penrose DM, Glick BR, Greenberg BM (2004) A multi-process phytoremediation system for removal of polycyclic aromatic hydrocarbons from contaminated soils. Environ Pollut 130:465–476
Joner EJ, Hirmann D, Szolar OHJ, Todorovic D, Leyval C, Loibner AP (2004) Priming effects on PAH degradation and ecotoxicity during a phytoremediation experiment. Envrion Pollut 128:429–435
Kipopoulou AM, Manoli E, Samara C (1999) Bioconcentration of polycyclic aromatic hydrocarbon vegetables grown in an industrial area. Environ Pollut 106:369–380
Labud V, Garcia C, Hernandez T (2007) Effect of hydrocarbon pollution on the microbial properties of a sandy and a clay soil. Chemosphere 66:1863–1871
Liu SL, Luo YM, Cao ZH (2004) Degradation of benzo[a]pyrene in soil with aebuscular mycorrhizal alfalfa. Environ Geochem Health 26:285–293
Macek T, Mackova M, Kas J (2000) Exploitation of plants for the removal of organics in environmental remediation. Biotechnol Adv 18:23–34
Margesin R, Walder G, Schinner F (2000) The impact of hydrocarbon remediation (diesel oil and polycyclic aromatic hydrocarbons) on enzyme activities and microbial properties of soil. Acta Biotechnol 20:313–333
Meng L, Qiao M, Hans PHA (2011) Phytoremediation efficiency of a PAH-contaminated industrial soil using ryegrass, white clover, and celery as mono- and mixed cultures. J Soils Sediments 10:1007–1016
Ni JZ, Luo YM, Wei R, Li XH (2008) Distribution patterns of polycyclic aromatic hydrocarbons among different organic carbon fractions of polluted agricultural soils. Geoderma1 46:277–282
Parrish ZD, Banks MK, Schwab AP (2004) Effectiveness of phytoremediation as a secondary treatment for polycyclic aromatic hydrocarbons (PAHs) in composted soil. Int J Phytoremediat 6(2):119–137
Phillips LA, Greer CW, Farrell RE, Germida JJ (2009) Field-scale assessment of weathered hydrocarbon degradation by mixed and single plant treatment. Appl Soil Ecol 42:7–17
Pilon-Smits E (2005) Phytoremediation. Ann Rev Plant Biol 56:15–39
Reilley K, Banks MK, Schwab AP (1996) Dissipation of polynuclear aromatic hydrocarbons in the rhizosphere. J Environ Qual 25:212–219
Singh J, Singh DK (2005) Dehydrogenase and phosphomonoesterase activities in groundnut (Arachis hypogaea L.) field after diazinon, imidacloprid and lindane treatments. Chemosphere 60:32–42
Singleton DR, Powell SN, Sangaiah R, Gold A, Ball LM, Aitken MD (2005) Stable-isotope probing of bacteria capable of degrading salicylate, naphthalene, or phenanthrene in a bioreactor treating contaminated soil. Appl Environ Micorbiol 71:1202–1209
Singleton DR, Powell SN, Sangaiah R, Gold A, Ball LM, Aitken MD (2006) Substrate preference of uncultivated PAH-degrading bacteria determined by simultaneous stable-isotope probing with phenanthene and pyrene. In: American Society for Microbiology, 106th general meeting, Florida
Susarla S, Medina VF, McCutcheon SC (2002) Phytoremediation: an ecological solution to organic chemical contamination. Ecol Eng 18:647–658
Teng Y, Luo YM, Sun MM (2010) Effect of bioaugmentation by Paracoccus sp. strain HPD-2 on the soil microbial community and removal of polycyclic aromatic hydrocarbons from an aged contaminated soil. Bioresour Technol 101:3437–3443
Tu C, Teng Y, Luo YM (2011) PCB removal, soil enzyme activities, and microbial community structures during the phytoremediation by alfalfa in field soils. J Soils Sediments 11(4):649–656
Vance ED, Brookes PC, Jenkinson DS (1987) An extraction method for measuring soil microbial biomass-C. Soil Biol Biochem 19:703–707
Wei SQ, Pan SW (2010) Phytoremediation for soils contaminated by phenanthrene and pyrene with multiple plant species. J Soils Sediments 10:886–894
Wilcke W (2007) Global patterns of polycyclic aromatic hydrocarbons (PAHs) in soil. Geoderma 141:157–166
Wrenn BA, Venosa AD (1996) Selective enumeration of aromatic and aliphatic hydrocarbon degrading bacteria by a most-probable-number procedure. Can J Microbiol 42:252–258
Yao HY, Xu JM, Huang CY (2003) Substrate utilization pattern, biomass and activity of microbial communities in a sequence of heavy metal-polluted paddy soils. Geoderma 115:139–148
Acknowledgments
This work was supported by grants from the Knowledge Innovation Program of the Chinese Academy of Sciences (project KSCX2-YW-G-071), the National High Technology Research and Development Program of China (863 Program; project 2007AA061101), and the Natural Science Foundation of Jiangsu Province (project BK2009016).
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Jaco Vangronsveld
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Sun, M., Fu, D., Teng, Y. et al. In situ phytoremediation of PAH-contaminated soil by intercropping alfalfa (Medicago sativa L.) with tall fescue (Festuca arundinacea Schreb.) and associated soil microbial activity. J Soils Sediments 11, 980–989 (2011). https://doi.org/10.1007/s11368-011-0382-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-011-0382-z