Abstract
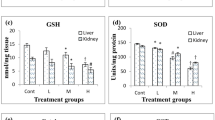
Arsenic (As) is a well-known contaminant of global groundwater. Its exposure causes several hazardous effects on animals and human via oxidative stress. The present study examined the effect of polydatin (PD) on free radical overproduction in rats exposed to As. Thirty-five male rats randomly allocated into five equal groups. To the control group, physiological saline was given orally and to the second group only 100 mg/L As was given by drinking water for 60 days. The other groups were treated with As (100 mg/L) and PD orally at 50, 100, and 200 mg/kg/day, respectively. Treatment with As enhanced malondialdehyde level but decreased glutathione level in blood, liver, kidney, brain, lung, and heart of rats. Also, As decreased superoxide dismutase and catalase activities of erythrocyte, liver, kidney, brain, lung, and heart in rats. Furthermore, As treatment gave rise to increased DNA damage and gene expressions of interleukin 1 beta (IL-1β), nuclear factor kappa beta (NFκB), p53, and tumor necrosis factor-α (TNF-α) in the lung, brain, kidney, and liver. However, treatment of PD ameliorated As-exposed lipid peroxidation, antioxidant enzymes activities, DNA damage, gene expressions, and histopathological changes in tissues. In conclusion, PD has a dose-dependent protective effect on lipid peroxidation and antioxidant defense mechanism in rats against As exposure.




Similar content being viewed by others
References
Aebi H (1974) Catalase in vitro. In: Bergmeyer U (ed) Methods of enzymatic analysis. Academic Press, New York and London, pp 673–677
Andrew AS, Warren AJ, Barchowsky A, Temple KA, Klei L, Soucy NV, O’Hara KA, Hamilton JW (2003) Genomic and proteomic profiling of responses to toxic metals in human lung cells. Environ Health Perspect 111(6):825–835
Balakumar BS, Ramanathan K, Kumaresan S, Suresh R (2010) DNA damage by sodium arsenite in experimental rats: ameliorative effects of antioxidant vitamins C and E. Indian J Sci Technol 3:322–327
Barchowsky A, Roussel RR, Klei LR, James PE, Ganju N, Smity KR, Dudek EJ (1999) Low levels of arsenic trioxide stimulate proliferatiove signals in primary vascular cells without activating stress effector pathways. Toxicol Appl Pharmacol 159:65–75
Beutler E, Duron O, Kelly BM (1993) Improved method for the determination of blood glutathione. J Lab Clin Med 61:882–888
Bouaziz H, Sefi M, de Lapuente J, Borras M, Zeghal N (2015) In vitro cytotoxic and genotoxic effects of arsenic trioxide on human keratinocytes. IJIRSE 9(3):231–234
Cui X, Li S, Shraim A, Kobayashi Y, Hayakawa T, Kanno S, Yamamot M, Hirano S (2004) Subchronic exposure to arsenic through drinking water alters expression of cancer-related genes in rat liver. Toxicol Pathol 32(1):64–72
Das S, Santra A, Lahiri S, Guha Mazumder DN (2005) Implications of oxidative stress and hepatic cytokine (TNF-a and IL-6) response in the pathogenesis of hepatic collagenesis in chronic arsenic toxicity. Toxicol Appl Pharmacol 204:18–26
Drabkin DL, Austin JH (1935) Spectrophotometric studies. II. Preparations from washed blood cells; nitric oxide hemoglobin and sulfhemoglobin. J Biol Chem 112:51–65
Draper HH, Hardley M (1990) Malondialdehyde determination as index of lipid peroxidation. Methods Enzymol 186:421–431
Ganapathy S, Li P, Fagman J, Yu T, Lafontant J, Zhang G, Chen C (2016) Low doses of arsenic, via perturbing p53, promotes tumorigenesis. Toxicol Appl Pharmacol 306:98–104
Ghosh A, Mandal AK, Sarkar S, Das N (2011) Hepatoprotective and neuroprotective activity of liposomal quercetin in combating chronic arsenic induced oxidative damage in liver and brain of rats. Drug Deliv 18:451–459
Gurr JR, Bau DT, Liu F, Lynn S, Jan KY (1999) Dithiothreitol enhances arsenic trioxide induced apoptosis in NB4 cells. Mol Pharmacol 56:102–109
Hei TK, Liu SX, Waldren C (1998) Mutagenecity of arsenic in mammalian cells: role of reactive oxygen species. Proc Natl Acad Sci 95:8103–8107
Hemalatha P, Reddy AG, Reddy YR, Shivakumar P (2013) Evaluation of protective effect of N-acetyl cysteine on arsenic-induced hepatotoxicity. J Nat Sci Biol Med 4:393–395
Huang ZS, Wang ZW, Liu MP, Zhong SQ, Li QM, Rong XL (1999) Protective effects of polydatin against CCl(4)-induced injury to primarily cultured rat hepatocytes. World J Gastroenterol 5(1):41–44
Ince S, Arslan-Acaroz D, Neuwirth O, Demirel HH, Denk B, Kucukkurt I, Turkmen R (2014a) Protective effect of polydatin, a natural precursor of resveratrol, against cisplatin-induced toxicity in rats. Food Chem Toxicol 72:147–153
Ince S, Kucukkurt I, Demirel HH, Arslan-Acaröz D, Akbel E, Cigerci IH (2014b) Protective effects of boron on cyclophosphamide induced lipid peroxidation and genotoxicity in rats. Chemosphere 108:197–204
Ince S, Kucukkurt I, Turkmen R, Demirel HH, Sever E (2013) Dietary Yucca schidigera supplementation reduces arsenic-induced oxidative stress in Swiss albino mice. Toxicol Ind Health 29:904–914
Jin Y, Sun G, Li X, Li G, Lu C, Qu L (2004) Study on the toxic effects induced by different arsenicals in primary cultured rat astroglia. Toxicol Appl Pharmacol 196:396–403
Kalendar R, Lee D, Schulman AH (2009) FastPCR software for PCR primer and probe design and repeat search. Genes, genomes and Genomics 3(1):1–14
Kocyigit A, Keles H, Selek S, Guzel S, Celik H, Erel O (2005) Increased DNA damage and oxidative stress in patients with cutaneous leishmaniasis. Mutat Res 585:71–78
Kucukkurt I, Ince S, Demirel HH, Turkmen R, Akbel E, Celik Y (2015) The effects of boron on arsenic-induced lipid peroxidation and antioxidant status in male and female rats. J Biochem Mol Toxicol 29(12):564–571
Li Z, Piao F, Liu S, Wang Y, Qu S (2010) Subchronic exposure to arsenic trioxide-induced oxidative DNA damage in kidney tissue of mice. Exp Toxicol Pathol 62:543–547
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Luck H (1955) Catalase. In: Bergmeyer HU (ed) Methods in analysis. Academy Press, London
Lynn S, Gurr JR, Lai HT, Jan KY (2000) NADH oxidase activation is involved in arsenite-induced oxidative DNA damage in human vascular smooth muscle cells. Cir Res 86:514–519
Mandal BK, Suzuki KT (2002) Arsenic round the world: a review. Talanta 58:201–235
Mishra D, Gupta R, Pant SC, Kushwah P, Satish HT, Flora SJ (2009) Co-administration of monoisoamyl dimercaptosuccinic acid and Moringa oleifera seed powder protects arsenic-induced oxidative stress and metal distribution in mice. Toxicol Mech Methods 19:169–182
Moore LE, Smith AH, Eng C, Kalman D, DeVries S, Bhargava V, Chew K, Moore D, Ferreccio C, Rey OA, Waldman FM (2002) Arsenic-related chromosomal alterations in bladder cancer. J Natl Cancer Inst 94:1688–1696
Muenyi CS, Ljungman M, States JC (2015) Arsenic disruption of DNA damage responses-potential role in carcinogenesis and chemotherapy. Biomol Ther 5(4):2184–2193
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Rea MA, Gregg JP, Qin Q, Phillips MA, Rice RH (2003) Global alteration of gene expression in human keratinocytes by inorganic arsenic. Carcinogenesis 24(4):747–756
Sankar P, Telang AG, Kalaivanan R, Karunakaran V, Suresh S, Kesavan M (2016) Oral nanoparticulate curcumin combating arsenic-induced oxidative damage in kidney and brain of rats. Toxicol Ind Health 32(3):410–421
Shan B, Cai YZ, Brooks JD, Corke H (2008) Antibacterial properties of Polygonum cuspidatum roots and their major bioactive constituents. Food Chem 109:530–537
Sinha M, Manna P, Sil PC (2008) Protective effect of arjunolic acid against arsenic-induced oxidative stress in mouse brain. J Biochem Mol Toxicol 22(1):15–26. https://doi.org/10.1002/jbt.20209
Sun Y, Oberley LW, Li Y (1988) A simple method for clinical assay of superoxide dismutase. Clin Chem 34:497–500
Winterbourn CC, Hawkins RE, Brain M, Carrell RW (1975) The estimation of red cell superoxide activity. J Lab Clin Med 55:337–341
Xie X, Peng J, Huang K, Huang J, Shen Shen X, Liu P, Huang H (2012) Polydatin ameliorates experimental diabetes-induced fibronectin through inhibiting the activation of NF-kB signaling pathway in rat glomerular mesangial cells. Mol Cell Endocrinol 362(1–2):183–193. https://doi.org/10.1016/j.mce.2012.06.008
Zheng XH, Watts GS, Vaught S, Gandolfi AJ (2003) Low-level arsenite induced gene expression in HEK293 cells. Toxicology 187(1):39–48
Funding
This study was supported by a grant from the Afyon Kocatepe University Scientific Research Council, Afyonkarahisar, Turkey (Project no: 15.VF.07).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Arslan-Acaroz, D., Zemheri, F., Demirel, H.H. et al. In vivo assessment of polydatin, a natural polyphenol compound, on arsenic-induced free radical overproduction, gene expression, and genotoxicity. Environ Sci Pollut Res 25, 2614–2622 (2018). https://doi.org/10.1007/s11356-017-0391-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-0391-6