Abstract
Purpose
We evaluated a dimeric RGD-peptide, [68Ga]DOTA-E-[c(RGDfK)]2, for positron emission tomography (PET) imaging of myocardial integrin expression associated with extracellular matrix remodeling after myocardial infarction (MI) in rat.
Procedures
Male Sprague-Dawley rats were studied at 7 days and 4 weeks after MI induced by permanent ligation of the left coronary artery and compared with sham-operated controls.
Results
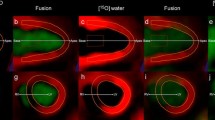
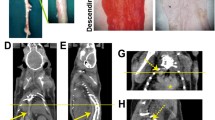
In vivo imaging revealed higher tracer uptake in the infarcted area than in the remote non-infarcted myocardium of the same rats both at 7 days (MI/remote ratio, 2.25 ± 0.24) and 4 weeks (MI/remote ratio, 2.13 ± 0.37) post-MI. Compared with sham-operated rats, tracer uptake was higher also in the remote, non-infarcted myocardium of MI rats both at 7 days and 4 weeks where it coincided with an increased interstitial fibrosis. Standardized uptake values correlated well with the results of tracer kinetic modeling. Autoradiography confirmed the imaging results showing 5.1 times higher tracer uptake in the infarcted than remote area. Tracer uptake correlated with the amount of β3 integrin subunits in the infarcted area.
Conclusions
Our results show that integrin-targeting [68Ga]DOTA-E-[c(RGDfK)]2 is a potential tracer for monitoring of myocardial extracellular matrix remodeling after MI using PET.




Similar content being viewed by others
References
Meoli DF, Sadeghi MM, Krassilnikova S et al (2004) Noninvasive imaging of myocardial angiogenesis following experimental myocardial infarction. J Clin Invest 113:1684–1691
Higuchi T, Bengel FM, Seidl S et al (2008) Assessment of alphavbeta3 integrin expression after myocardial infarction by positron emission tomography. Cardiovasc Res 78:395–403
van den Borne SW, Diez J et al (2010) Myocardial remodeling after infarction: the role of myofibroblasts. Nat Rev Cardiol 7:30–37
van den Borne SW, Isobe S, Verjans JW et al (2008) Molecular imaging of interstitial alterations in remodeling myocardium after myocardial infarction. J Am Coll Cardiol 52:2017–2028
Sherif HM, Saraste A, Nekolla SG et al (2012) Molecular imaging of early alphavbeta3 integrin expression predicts long-term left-ventricle remodeling after myocardial infarction in rats. J Nucl Med 53:318–323
Dijkgraaf I, Kruijtzer JA, Liu S et al (2007) Improved targeting of the alphavbeta3 integrin by multimerisation of RGD peptides. Eur J Nucl Med Mol Imaging 34:267–273
Dijkgraaf I, Yim CB, Franssen GM et al (2011) PET imaging of alphavbeta3 integrin expression in tumours with 68Ga-labelled mono-, di- and tetrameric RGD peptides. Eur J Nucl Med Mol Imaging 38:128–137
Dijkgraaf I, Terry SY, McBride WJ et al (2013) Imaging integrin alpha-v-beta-3 expression in tumors with an 18 F-labeled dimeric RGD peptide. Contrast Media Mol Imaging 8:238–245
Hua J, Dobrucki LW, Sadeghim MM et al (2005) Noninvasive imaging of angiogenesis with a 99mTc-labeled peptide targeted at alphavbeta3 integrin after murine hindlimb ischemia. Circulation 111:3255–3260
Gao H, Lang L, Guo N et al (2012) PET imaging of angiogenesis after myocardial infarction/reperfusion using a one-step labeled integrin-targeted tracer 18 F-AlF-NOTA-PRGD2. Eur J Nucl Med Mol Imaging 39:683–692
Menichetti L, Kusmic C, Panetta D et al (2013) MicroPET/CT imaging of αvβ3 integrin via a novel 68Ga-NOTA-RGD peptidomimetic conjugate in rat myocardial infarction. Eur J Nucl Med Mol Imaging 40:1265–1274
Laitinen I, Notni J, Pohle K et al (2013) Comparison of cyclic RGD peptides for αvβ3 integrin detection in a rat model of myocardial infarction. EJNMMI Res 3:38
Pfeffer MA, Pfeffer JM, Fishbein MC et al (1979) Myocardial infarct size and ventricular function in rats. Circ Res 44:503–512
Sherif HM, Saraste A, Weidl E et al (2009) Evaluation of a novel 18 F-labeled positron-emission tomography perfusion tracer for the assessment of myocardial infarct size in rats. Circ Cardiovasc Imaging 2:77–84
Dijkgraaf I, Rijnders AY, Soede A et al (2007) Synthesis of DOTA-conjugated multivalent cyclic-RGD peptide dendrimers via 1,3-dipolar cycloaddition and their biological evaluation: implications for tumor targeting and tumor imaging purposes. Org Biomol Chem 5:935–944
Dijkgraaf I, Kruijtzer JA, Frielink C et al (2006) Synthesis and biological evaluation of potent alphavbeta3-integrin receptor antagonists. Nucl Med Biol 33:953–961
Seppälä J, Seppänen M, Arponen E, Lindholm P, Minn H (2009) Carbon-11 acetate PET/CT based dose escalated IMRT in prostate cancer. Radiother Oncol 93:234–240
Haukkala J, Laitinen I, Luoto P et al (2009) 68Ga-DOTA-RGD peptide: biodistribution and binding into atherosclerotic plaques in mice. Eur J Nucl Med Mol Imaging 36:2058–2067
Laitinen I, Saraste A, Weidl E et al (2009) Evaluation of alphavbeta3 integrin-targeted positron emission tomography tracer 18 F-galacto-RGD for imaging of vascular inflammation in atherosclerotic mice. Circ Cardiovasc Imaging 2:331–338
Silvola JM, Saraste A, Forsback S et al (2011) Detection of hypoxia by [18 F]EF5 in atherosclerotic plaques in mice. Arterioscler Thromb Vasc Biol 31:1011–1015
Zhang X, Xiong Z, Wu Y et al (2006) Quantitative PET imaging of tumor integrin alphavbeta3 expression with 18 F-FRGD2. J Nucl Med 47:113–121
Kim JH, Kim YH, Kim YJ et al (2013) Quantitative positron emission tomography imaging of angiogenesis in rats with forelimb ischemia using 68Ga-NOTA-c(RGDyK. Angiogenesis 16:837–846
Manso AM, Kang SM, Ross RS (2009) Integrins, focal adhesions, and cardiac fibroblasts. J Investig Med 57:856–860
Kuppuswamy D, Kerr C, Narishige T et al (1997) Association of tyrosine-phosphorylated c-Src with the cytoskeleton of hypertrophying myocardium. J Biol Chem 272:4500–4508
Higuchi T, Wester HJ, Schwaiger M (2007) Imaging of angiogenesis in cardiology. Eur J Nucl Med Mol Imaging 34:S9–S19
van den Borne SW, Isobe S, Zandbergen HR et al (2009) Molecular imaging for efficacy of pharmacologic intervention in myocardial remodeling. J Am Coll Cardiol 2:187–198
Acknowledgments
The authors thank Erica Nyman and Liisa Lempiäinen for performing tissue sectioning and immunohistochemistry, Ville Aalto, M.Sc., with the CoE in Molecular Imaging, for the advice on the statistical analysis; Juho Virtanen, M.Sc., with the Turku PET Centre, for Image-J automatic analysis scripts; and Robert M. Badeau, Ph.D., with the University of Turku Language Center, for English language proofreading. The studies were conducted within the Finnish Centre of Excellence in Cardiovascular and Metabolic Diseases supported by the Academy of Finland, the University of Turku, the Turku University Hospital, and the Åbo Akademi University. This study was financially supported by the Turku University Hospital, Sigrid Juselius Foundation, Finnish Cultural Foundation, and Finnish Foundation for Cardiovascular Research.
Conflict of Interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
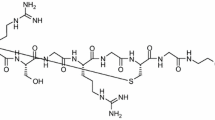
Descriptions of the methods used in present study are described in more detail in the Supplementary Material available online. Supplementary Material also contains structure of the peptide used in this study (Fig. S1), detailed biodistribution results (Fig. S2), and the results of using skeletal muscle as a reference tissue. (DOCX 889 kb)
Rights and permissions
About this article
Cite this article
Kiugel, M., Dijkgraaf, I., Kytö, V. et al. Dimeric [68Ga]DOTA-RGD Peptide Targeting αvβ3 Integrin Reveals Extracellular Matrix Alterations after Myocardial Infarction. Mol Imaging Biol 16, 793–801 (2014). https://doi.org/10.1007/s11307-014-0752-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-014-0752-1