Abstract
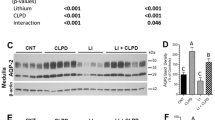
Previously, we localized ADP-activated P2Y12 receptor (R) in rodent kidney and showed that its blockade by clopidogrel bisulfate (CLPD) attenuates lithium (Li)-induced nephrogenic diabetes insipidus (NDI). Here, we evaluated the effect of prasugrel (PRSG) administration on Li-induced NDI in mice. Both CLPD and PRSG belong to the thienopyridine class of ADP receptor antagonists. Groups of age-matched adult male B6D2 mice (N = 5/group) were fed either regular rodent chow (CNT), or with added LiCl (40 mmol/kg chow) or PRSG in drinking water (10 mg/kg bw/day) or a combination of LiCl and PRSG for 14 days and then euthanized. Water intake and urine output were determined and blood and kidney tissues were collected and analyzed. PRSG administration completely suppressed Li-induced polydipsia and polyuria and significantly prevented Li-induced decreases in AQP2 protein abundance in renal cortex and medulla. However, PRSG either alone or in combination with Li did not have a significant effect on the protein abundances of NKCC2 or NCC in the cortex and/or medulla. Immunofluorescence microscopy revealed that PRSG administration prevented Li-induced alterations in cellular disposition of AQP2 protein in medullary collecting ducts. Serum Li, Na, and osmolality were not affected by the administration of PRSG. Similar to CLPD, PRSG administration had no effect on Li-induced increase in urinary Na excretion. However, unlike CLPD, PRSG did not augment Li-induced increase in urinary arginine vasopressin (AVP) excretion. Taken together, these data suggest that the pharmacological inhibition of P2Y12-R by the thienopyridine group of drugs may potentially offer therapeutic benefits in Li-induced NDI.







Similar content being viewed by others
References
Rieg T, Bundey RA, Chen Y, Deschens G, Junger W, Insel PA, Vallon V (2007) Mice lacking P2Y2 receptors have salt-resistant hypertension and facilitated renal Na+ and water reabsorption. FASEB J 21:3717–3726. doi:10.1096/fj.07-8807com
Zhang Y, Sands JM, Kohan DE, Nelson RD, Martin CF, Carlson NG, Kamerath CD, Ge Y, Klein JD, Kishore BK (2008) Potential role of purinergic signaling in urinary concentration in inner medulla: insights from P2Y2 receptor gene knockout mice. Am J Physiol Renal Physiol 295:F1715–F1724. doi:10.1152/ajprenal.90311.2008
Wildman SS, Marks J, Turner CM, Yew-booth L, Peppiat-Wildman CM, King BF, Shirley DG, Wang W, Unwin RJ (2008) Sodium-dependent regulation of renal amiloride-sensitive currents by apical P2 receptors. J Am Soc Nephrol 19:731–742. doi:10.1681/ASN.2007040443
Stockand JD, Mironova E, Bugaj V, Rieg T, Insel PA, Vallon V, Peti-Peterdi J, Pochynyuk O (2010) Purinergic inhibition of ENaC produces aldosterone escape. J Am Soc Nephrol 21:1903–1911. doi:10.1681/ASN.2010040377
Vallon V (2008) P2 receptors in the regulation of renal transport mechanisms. Am J Physiol Renal Physiol 294:F10–F27. doi:10.1152/ajprenal.00432.2007
Kishore BK, Nelson RD, Miller RL, Carlson NG, Kohan DE (2009) P2Y2 receptors and water transport in the kidney. Puriner Signal 5:491–499. doi:10.1007/s11302-009-9151-5
Prætorius H, Leipziger J (2010) Intrarenal purinergic signaling in the control or renal tubular transport. Annu Rev Physiol 72:377–393. doi:10.1146/annurev-physiol-021909-135825
Vallon V, Rieg T (2011) Regulation of renal transport mechanisms. Am J Physiol Renal Physiol 301:F463–F475
Leipziger J (2011) Luminal nucleotides are tonic inhibitors of renal tubular transport. Curr Opin Nephrol Hyperten 20:518–522. doi:10.1097/MNH.0b013e3283487393
Zhang Y, Peti-Peterdi J, Müller CE, Carlson NG, Baqi Y, Strasburg DL, Heiney KM, Villanueva K, Kohan DE, Kishore BK (2015) P2Y12 receptor localizes in the renal collecting duct and its blockade augments arginine vasopressin action and alleviates nephrogenic diabetes insipidus. J Am Soc Nephrol 26:2978–2987. doi:10.1681/ASN.2014010118
Zhang Y, Nelson RD, Carlson NG, Kamerath CD, Kohan DE, Kishore BK (2009) Potential role of purinerigic signaling in lithium-induced nephrogenic diabetes insipidus. Am J Physiol Renal Physiol 296:F1194–F1201. doi:10.1152/ajprenal.90774.2008
Grünfeld JP, Rossier BC (2009) Lithium nephrotoxicity revisited. Nat Rev Nephrol 5:270–276. doi:10.1038/nrneph.2009.43
Kishore BK, Ecelbarger CM (2013) Lithium: a versatile tool for understanding renal physiology. Am J Physiol Renal Physiol 304:F1139–F1149. doi:10.1152/ajprenal.00718.2012
Zhang Y, Pop IL, Carlson NG, Kishore BK (2012) Genetic deletion of the P2Y2 receptor offers significant resistance to development of lithium-induced polyuria accompanied by alterations in PGE2 signaling. Am J Physiol Renal Physiol 302:F70–F77. doi:10.1152/ajprenal.00444.2011
Zhang Y, Li L, Kohan DE, Ecelbarger CM, Kishore BK (2013) Attenuation of lithium-induced natriuresis and kaliuresis in P2Y2 receptor knockout mice. Am J Physiol Renal Physiol 305:F407–F416. doi:10.1152/ajprenal.00464.2012
Zhang Y, Peti-Peterdi J, Heiney KM, Riquier-Brison A, Carlson NG, Müller CE, Ecelbarger CM, Kishore BK (2015) Clopidogrel attenuates lithium-induced alterations in renal water and sodium channels/transporters in mice. Puriner Signal 11:507–518. doi:10.1007/s11302-015-9469-0
Sangkuhl K, Klein TE, Altman RB (2010) Clopidogrel pathway. Pharmacogenet Genomics 20:463–465. doi:10.1097/FPC.0b013e3283385420
Savi P, Zachayus J-L, Delesque-Touchard N, Labouret C, Herve C, Uzbiaga M-F, Pereillo J-M, Culouscou J-M, Bono F, Ferrara P, Herbert J-M (2006) The active metabolite of clopidogrel disrupts P2Y12 receptor oligomers and partitions them out of lipid rafts. Proc Natl Acad Sci U S A 103:11069–11074. doi:10.1073/pnas.0510446103
Wallentin L (2009) P2Y12 inhibitors: differences in properties and mechanisms of action and potential consequences for clinical use. Eur Heart J 30:1964–1977. doi:10.1093/eurheartj/ehp296
Giorgi MA, Cohen Arazi H, Gonzalez CD, Di Girolamo G (2011) Beyond efficacy: pharmacokinetic differences between clopidogrel, prasugrel and ticagrel. Expert Opin Pharmacother 12:1285–1295. doi:10.1517/14656566.2011.550573
Savi P, Pereillo JM, Uzabiaga ME, Combalbert J, Picard C, Maffrand JP, Pascal M, Herbert JM (2000) Identification and biological activity of the active metabolite of clopidogrel. Thromb Haemost 84:891–896
Brophy JM, Babapulle MN, Costa V, Rinfret S (2006) A Pharmacoepidemiology study of the interaction between atorvastatin and clopidogrel after percutaneous coronary intervention. Am Heart J 152:263–269. doi:10.1016/j.ahj.2005.08.023
Berniochner I, Sibbing D (2012) Thienopyridine and other ADP-receptor antagonists. Handb Exp Pharmacol 210:165–198. doi:10.1007/978-3-642-29423-5_7
Wallentin L, Vaenhorst C, James S, Erlinge D, Braun Ö, Jakubowski JA, Sugidachi A, Winters KJ, Siebahn A (2008) Prasugrel achieves greater and faster P2Y12 receptor-mediated platelet inhibition than clopidogrel due to more efficient generation of its active metabolite in aspirin-treated patients with coronary artery disease. Eur Heart J 29:21–30. doi:10.1093/eurheartj/ehm545
Norgard NB, Abu-Fadel M (2009) Comparison of prasugrel and clopidogrel in patients with acute coronary syndrome undergoing percutaneous coronary intervention. Vasc Health Risk Manag:873–882
Hagihara K, Kazui M, Kurihara A, Yoshiike M, Honda K, Okazaki O, Farid NA, Ikeda T (2009) A possible mechanism for the differences in efficiency and variability of active metabolite formation from thienopyridine antiplatelet agents, prasugrel and clopidogrel. Drug Met Disp 17:2145–2152. doi:10.1124/dmd.109.028498
Reagan-Shaw S, Nihal M, Ahmad N (2007) Dose translation from animal to human studies revisited. FASEB J 22:659–661. doi:10.1096/fj.07-9574LSF
Hanner F, Lan L, Nguyen TX, Yu A, Peti-Peterdi J (2012) Intrarenal localization of the plasma membrane channel pannexin 1. Am J Physiol Renal Physiol 303:F1454–F1459. doi:10.1152/ajprenal.00206.2011
Baldenssarini RJ, Pompili M, Tondo L (2006) Suicide in bipolar disorder: risks and management. CNS Spectr 11:465–471
Cipriani A, Hawton K, Stockton S, Geddes JR (2013) Lithium in the prevention of suicide in mood disorders: updated systemic review and meta-analysis. BMJ 346:f3646. doi:10.1136/ bmj.f3646
Geddes J, Miklowitz DJ (2013) Treatment of bipolar disorder. Lancet 381:1672–1682. doi:10.1016/S0140-6736(13)60857-0
Rowe MK, Chuang DM (2004) Lithium neuroprotection: molecular mechanisms and clinical implications. Expert Rev Mol Med 18:1–18. doi:10.1017/S1462399404008385
Wade A, Yokoo H, Yanagita T, Kobayashi H (2005) Lithium: potential therapeutics against acute brain injuries and chronic neurodegenerative diseases. J Pharmacol Sc 99:307–321
Florenza OV, de Paula VJ, Machado-Vieira R, Diniz BS, Gattaz WF (2012) Does lithium prevent Alzheimer’s disease? Drugs Aging 29:335–342. doi:10.2165/11599180-000000000-00000
Chiu CT, Want Z, Hunsberger JG, Chuang DM (2013) Therapeutic potential of mood stabilizers lithium and valproic acid: beyond bipolar disorder. Pharmacol Rev 65:105–142. doi:10.1124/pr.111.005512
Luckey AE, Parsa CJ (2003) Fluid and electrolytes in the aged. Arch Sug 138:1055–1060. doi:10.1001/ archsurg.138.10.1055
Rej S, Herrmann N, Shulman K (2012) The effects of lithium on renal function in older adults—a systematic review. J Geriatr Psychiatry Neurol 25:51–61. doi:10.1177/0891988712436690
Bedford JJ, Leader JP, Jing R, Walker LJ, Klein JD, Sands JM, Walker RJ (2008) Amiloride restores renal medullary osmolytes in lithium-induced nephrogenic diabetes insipidus. Am J Physiol Renal Physiol 294:F812–F820. doi:10.1152/ajprenal.00554.2007
Kortenoeven ML, Li Y, Shaw S, Gaeggeler HP, Rossier BC, Wetzels JF, Deen PM (2009) Amiloride blocks lithium entry through sodium channel thereby attenuating the resultant nephrogenic diabetes insipidus. Kidney Int 76:44–53. doi:10.1038/ki.2009.91
Finley PR, Warner D, Peabody CA (1995) Clinical relevance of drug interactions with lithium. Clin Pharmacokinet 29:172–191. doi:10.2165/00003088-199529030-00004
Murray MD, Brater DC (1993) Renal toxicity of the nonsteroidal anti-inflammatory drugs. Annu Rev Pharmacol Toxicol 32:435–465. doi:10.1146/annurev.pa.33.040193.002251
Phelan KM, Mosholder AD, Lu S (2003) Lithium interaction with the cyclooxygenase-2 inhibitors reofecoxib and celecoxib and other nonsteroidal anti-inflammatory drugs. J Clin Psychiatry 64:1328–1334
Conaghan PG (2012) A turbulent decade of NSAIDs: update on current concepts of classification, epidemiology, comparative efficacy, and toxicity. Rheumatol Int 32:1491–1502. doi:10.1007/s00296-011-2263-6
Kishore BK, Brandes AU, Carlson NG, Zhang Y (2016) Prasugrel suppresses development of lithium-induced nephrogenic diabetes insipidus in mice (abstract). FASEB J 30:1220.5
Acknowledgements
This work was supported by a grant from the US Department of Veterans Affairs Merit Review Program (to B. K. Kishore), the resources and facilities at the VA SLC Health Care System, Salt Lake City, Utah, and Marriott Cardiovascular Fellowship (to C. M. Ecelbarger). A. Brandes has been supported by Undergraduate Research Opportunities Program (UROP) of the University of Utah. Additional funding sources include National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-64324 (to J. Peti-Peterdi), and an Established Investigator Award from the American Heart Association (to C. M. Ecelbarger). The authors thank Kristina M. Heiney and Hwal Lee for the technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Yue Zhang declares that s/he has no conflict of interest.
János Peti-Peterdi declares that s/he has no conflict of interest.
Anna U. Brandes declares that s/he has no conflict of interest.
Anne Riquier-Brison declares that s/he has no conflict of interest.
Noel G. Carlson declares that s/he has no conflict of interest.
Christa E. Müller declares that s/he has no conflict of interest.
Carolyn M. Ecelbarger declares that s/he has no conflict of interest.
Bellamkonda K. Kishore declares that s/he has no conflict of interest.
Parts of this work were presented at the Experiment mal Biology 2016 meeting organized by FASEB, April 2016 in San Diego, CA, and appeared as a printed abstract in the proceedings of that meeting [44].
Ethical approval
The animal procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of the Veterans Affairs Salt Lake City Health Care System, Salt Lake City, Utah.
Rights and permissions
About this article
Cite this article
Zhang, Y., Peti-Peterdi, J., Brandes, A.U. et al. Prasugrel suppresses development of lithium-induced nephrogenic diabetes insipidus in mice. Purinergic Signalling 13, 239–248 (2017). https://doi.org/10.1007/s11302-017-9555-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11302-017-9555-6