Abstract
It is not clear how the increase in intraluminal pressure behind an obstructing ureteric calculus causes an increase in action potential frequency in ureteric sensory nerves so the pain messages are transmitted to the brain. It has been proposed that ureteric distension causes urothelial release of ATP, which activates purinoceptors on suburothelial nociceptive sensory nerves. The purpose of this study was to determine whether distension of the human ureter results in the release of ATP and whether the nociceptive P2 receptor, P2X3, is expressed on suburothelial sensory nerves in the human ureter. Human ureter segments were perfused with Krebs solution and intermittently distended to a range of pressures. Samples of perfusate were collected throughout and the ATP concentration ([ATP]) was determined using a luciferin-luciferase assay. Sections of ureter were stained using antibodies against P2X3 and capsaicin receptors (TRPV1). [ATP] rose to more than 10 times baseline levels after distension beyond a threshold of 25–30 cmH2O. Immunofluorescence studies on consecutive frozen sections showed that suburothelial nerves stained positively for P2X3 and capsaicin receptors, with no staining in controls. These findings are consistent with the hypothesis that purinergic signalling is involved in human ureteric mechanosensory transduction, leading to nociception.
Similar content being viewed by others
Introduction
Pain due to a calculus causing an acute obstruction in the ureter can be severe and treatment with non-steroidal anti-inflammatory drugs or opiate analgesia does not always result in adequate pain relief. Human studies have shown that, following obstruction, continued urine production gradually increases the intraluminal pressure behind the blockage. The onset of pain occurs once the pressure reaches a mean threshold of 33 mmHg [1]. The pressure rise results in distension of the proximal ureter and renal pelvis, but it is not clear how this leads to neuronal activity so that pain messages might be transmitted to the brain. Local inflammation caused by the calculus releases inflammatory mediators, such as bradykinin or neurokinin A, which may sensitise afferent neurons via specific receptors [2–4]. However, the clear on-off link between pressure and nerve activity in animal studies and pressure and pain in human studies strongly indicate that the release of inflammatory mediators is not the principal mechanosensory transduction mechanism [1, 5]. There is a rich plexus of suburothelial afferent nerves in the ureter [6], and the afferent pathways from here via the splanchnic plexi, lower lumbar dorsal nerve roots and spinothalamic tracts have been well documented and studied [5, 7].
Burnstock [8] proposed that in tubes (e.g. ureter, salivary duct, bile duct, vagina and intestine) and in sacs (e.g. urinary bladder, gall bladder and lung) nociceptive mechanosensory transduction occurs where distension releases ATP from the epithelial cells lining these organs, which then activates P2X3 and/or P2X2/3 receptors on subepithelial sensory nerve plexuses to relay messages to the central nervous system pain centres. Evidence to support the mechanosensory role of ATP was first shown in the bladder [9, 10]. P2X3 receptor immunoreactivity has been found on nerves in the suburothelial plexus in the rat bladder [11]. Increased electrical activity was recorded in the afferent pelvic nerves of rats during slow distension of the bladder, and this activity was inhibited by desensitising the P2X3 receptors with α,β-meATP and also by receptor antagonism with suramin [12]. There was also reduced sensory nerve activity in P2X3 receptor-deficient mice [13]. ATP was shown to be released during distension of the mouse bladder, proportionate to the degree of distension and corresponding to the activity of multi-fibre pelvic nerve afferents [14].
In a later study, it was shown that distension of the guinea-pig ureter increased spike discharge in sensory neurons, which was mimicked by ATP and reduced by ATP antagonists [15]. Knight et al. [16] found that distending the guinea-pig ureter caused a pressure-dependent release of ATP, approximately 10 times the basal release levels. Lee et al. [11] demonstrated P2X3 receptor immunoreactivity in the nerves in the suburothelial plexus in rats.
In the present study, experiments have been carried out to investigate whether ATP is released from the human ureter upon distension and whether human ureteric suburothelial sensory nerves express P2X3 receptors.
Materials and methods
Tissue
Following ethical approval and consent, 3-cm segments of ureter (of the proximal or mid third; the orientation being marked by a tread) were taken from patients undergoing radical nephrectomy for renal cell carcinoma, the sample as far from the kidney to be considered ‘normal’ tissue. The ureteric segments (which all appeared healthy and non-distended) were each cut into a 1-cm and a 2-cm length. The 1-cm segments were mounted on labelled cork squares in OCT embedding compound (BDH Laboratory supplies, Poole, UK) and frozen in isopentane pre-cooled in liquid nitrogen. lmmunohistochemical analysis was subsequently performed on these samples as described below. The remaining segments were orientated for use in the ATP release experiments so that perfusion was in the direction of peristalsis.
Immunofluorescence
Immunofluorescence was performed using antibodies against the P2X3 receptor and the capsaicin receptor (also known as the transient receptor potential vanilloid 1-TRPV1) on adjacent sections. Negative controls were performed in the absence of primary antibody and also with pre-adsorption of primary antibody with nascent peptide.
Tissues were sectioned at 12 μm on a cryostat (Reichert Jung CM1800) and collected on gelatin-coated slides and air-dried at room temperature for 30 min. For amplification a primary and secondary antibody with an avidin-biotin complex technique (ABC) was used as follows: the sections were fixed in 4% buffered formaldehyde for 2 min and then washed three times (each for 5 min) in phosphate-buffered saline (PBS). The sections were then incubated for 20 min at room temperature in 10% normal horse serum (NHS) in PBS + 0.05% merthiolate (Sigma Chemical Co., Poole, UK). Following this, sections were incubated overnight in primary antibody diluted 1:200 in 10% NHS in PBS + merthiolate. Incubation was performed in moisturised air-tight incubation chambers at room temperature. Incubation was also tried at 4°C for 3 days, with differing NaCl levels in the diluents, and with altered concentrations of primary antibody, but binding was not found to be more specific. The following day the sections were initially washed three times in PBS. They were then incubated for 1 h at room temperature in biotinylated donkey anti-rabbit immunoglobin (Jackson Immunoresearch Laboratories, Luton, UK). This was diluted 1:500 in 1% NHS in PBS and merthiolate. Following three further washes in PBS the sections were incubated in streptavidin FITC (fluorescein isothiocyanate; Jackson Immunoresearch Laboratories, Luton, UK) diluted 1:200 in PBS and merthiolate. This was performed at room temperature in the dark. The sections were then mounted under coverslips in a glycerol mountant (Citifluor Ltd, London, UK) and viewed using a Zeiss Axioplan (Oberkochen, Germany) microscope with a dark field linked to a Leica DC200 digital camera (Heerbrugg, Switzerland). A primary antibody against P2X3 receptors was used. The antibody was obtained through collaboration with Roche Bioscience (Palo Alto, CA, USA) and prepared according to methods described by Lee et al. [11]. Controls were performed on adjacent ureteric sections by omitting the primary antibody and also by pre-adsorbing the P2X3 antibody with its cognate peptide.
A significant amount of autofluorescence originated from the collagen fibres in the sections. This was dampened by a 5-min incubation of all sections in pontamine sky blue before mounting in glycerol mountant (Citifluor Ltd, London, UK). The sections were viewed in a Zeiss Axioplan microscope using a dark field linked to a Leica DC200 digital camera.
Measurement of ATP release from the human ureter during distension
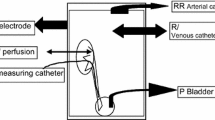
The 2-cm ureteric segments were catheterised at each end with a 6 French paediatric feeding tube and securely tied with 5/0 silk. They were placed in a Petri dish embedded with dental wax, into which non-corrodible metal staples had been previously positioned to keep the ureters straight and prevent excessive movement. The Petri dish was filled with Krebs solution, bubbled continuously with 95% O2 and 5% CO2. All experiments were carried out at room temperature as in the study by Knight et al. [16] (and personal communication). The feeding tube at the proximal end of the ureter was connected via a Luer lock to a 3-way tap. A diaphragm pressure transducer (model P23 ID, Gould Inc, Oxnard, CA, USA) was connected to one outlet and to the other (via further tubing) a peristaltic pump (Watson-Marlow Ltd, Fainmouth, UK). The feeding tube attached to the distal end of the ureter was cut short to minimise dead space and allowed to drain into a collecting container. Pressure was monitored using a Grass polygraph (model 7D). Pressure readings were calibrated and zeroed by altering the height of the preparation against a ruler. The segments were perfused in an isoperistaltic direction at a constant flow rate of 0.6 ml-1min with Krebs solution. Ureters were allowed to equilibrate for at least 45 min with perfusion before the experiment commenced. The experimental set-up allowed collection of 30-s perfusate aliquots in 0.5 ml Eppendorf tubes at various points during the experiments. Distensions were performed by clamping the feeding tube at the distal end of the ureter with a clip while continuing the pump flow until the desired pressure was achieved. The pump was switched off at this point. The haemostat was released after 1 min, the pump immediately restarted and the perfusates collected.
After equilibration, three baseline aliquots of perfusate were collected without distension. Distensions were performed in varying sequences to pressures in the range of 10–250 cmH2O. For each distension 30-s perfusate aliquots were collected for immediately before distension and at release + 30 s, + 60 s, + 90 s, + 150 s, + 270 s (n = at least 7 for each distension pressure, from 20 ureters). Distensions were separated by 12 min. Each sample was sealed in an Eppendorf tube and immediately frozen to −20°C for analysis as a batch.
The amount of ATP in the perfusate was quantified using the luciferin-luciferase assay. The perfusate samples (50 μl of each) and ATP standards were pipetted into a white, non-phosphorescent 96-well plate. The plate was placed in a luminometer (Lucy 1, Anthos Labtec, Salzburg, Austria) and processed automatically by injection of 100 μl of luciferin-luciferase reagent (ATP monitoring reagent, Bio-Orbit, Turku, Finland) into each well and measured for 10 s. The ATP concentrations ([ATP]) were calculated from a calibration curve constructed from the ATP standards. The detection limit was approximately 5 fmol per sample.
Statistical analysis
The concentration of ATP measured in each aliquot was plotted against time for each series of distensions (n ≥ 7 for each distension). The [ATP] in perfusate was also plotted grouping data into 30 cmH2O pressure ranges. At each pressure range a Student’s t-test was performed to compare mean [ATP] in control and following distension. [ATP] in perfusate following each distension was then plotted against distension pressure and a best-fit line plotted using linear regression to test whether the gradient of the line was significantly different from zero (i.e. whether the ATP release was pressure dependent). P < 0.05 was considered significant.
Results
Immunofluorescence
Nerves bundles were identified running immediately under the urothelium. These demonstrated immunoreactivity for both the P2X3 and TRPV1 receptors (Fig. 1a,b). Staining was absent following pre-adsorption of the anti-P2X3 receptor antibody with nascent peptide. No staining was seen in the absence of primary antibody (Fig. 1c,d).
Immunofluorescent staining of the suburothelial ureteric nerves. a P2X3 receptor. b Capsaicin receptor (TRPV1). c P2X3 receptor pre-absorption control. d No primary antibody control. Scale bar = 50 μm for each figure. U urothelium, S submucosa, arrows indicate suburothelial layer, arrowheads indicate sensory nerves
ATP release studies
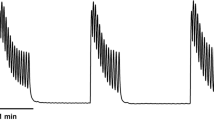
The mean baseline [ATP] was 1.4 + 0.18 nM. Increased concentrations of ATP were found in the perfusate following distension beyond a pressure threshold of 25–30 cmH2O in all ureter specimens. [ATP] rose to more than 10 times the baseline concentrations on distension. A typical experiment is demonstrated in Fig. 2, showing ATP concentrations as sequentially higher distension pressures (NB: The order of pressures was varied in different ureteric preparations, with a minimum of n = 7 for each pressure).
Figure 3 shows the [ATP] in perfusate immediately before and after distension grouped in pressure ranges. The mean [ATP] after distension was significantly greater than before distension in each pressure range (paired Student’s t-test, P < 0.01). Beyond the threshold there was a trend of increasing [ATP] release with higher pressures, but this difference was not statistically significant.
Discussion
The immunofluorescence study has shown that nerve bundles under the urothelium, which expressed TRPV1-like immunoreactivity (indicating that they are sensory afferent nerves), also expressed P2X3 receptor immunoreactivity. The specificity of the staining has been confirmed by the absence of staining in the absence of primary antibody and also when the primary antibody has been pre-adsorbed using nascent peptide. These data are consistent with the findings that P2X3 receptors may be expressed on nerves in the suburothelial ureter plexus in rats [11].
Release of ATP from guinea-pig ureter during distension has been described [16]. The present distension experiments have shown that the human ureter also releases ATP in response to distension, in excess of 10 times the basal release rate. While ATP is an agonist at both P2X1 receptors (found on smooth muscle) and P2X3 receptors, there is no evidence that ATP acts directly on smooth muscle receptors in the human ureter to cause a contraction, although very high (0.1 mM) concentrations of ATP have been shown to cause small contractions of the pig ureter [17]. Knight et al. [16] demonstrated in the guinea-pig that the release of ATP was abolished by removal of the urothelium and significantly reduced by monensin, brefeldin A or calcium depletion. Monensin is an inhibitor of vesicle formation from the Golgi apparatus [18], and brefeldin A disrupts vesicular trafficking by inhibiting protein transport from the endoplasmic reticulum to the Golgi apparatus [19]. This indicates that ATP is probably released from the urothelium by vesicular exocytosis (a calcium-dependent process). The release of ATP only occurred above a threshold of approximately 25–30 cmH2O. This is similar to the ureteric pressure threshold for pain measured by Risholm in 1954 [1]. He inserted ureteric catheters with an inflatable balloon close to their tips into 69 women undergoing cystoscopy for unrelated indications (using only urethral local anaesthetic gel as analgesia). The balloons were inflated in various parts of their ureters to simulate obstructing calculi and the pressures behind the obstructions were monitored. Inflation of the balloon was generally not painful, but as normal diuresis caused the pressures to gradually rise behind the obstruction the patients experienced pain similar to renal colic once a mean threshold of 33 mmHg (range: 21–58 mmHg) was reached. This is also consistent with the threshold for inducing a reflex fall in blood pressure in spinally injured cats which was a ureteric pressure of 25–30 mmHg [20]. Additionally, Sann and Cervero [21] found that approximately half of the units in the guinea-pig afferent ureteric nerves, which responded to ureteric pressure changes, had a threshold of over 20 mmHg. It was suggested that these nerves are more likely to conduct pain messages, whilst the nerves with lower pressure thresholds responded to the lower pressures generated by normal peristalsis and may be involved in a peristalsis feedback control mechanism. In the guinea-pig ureter beyond the threshold pressure, ATP release was clearly pressure dependent [16]. We found that although the mean [ATP] in the perfusate was higher at higher pressures, the differences were not statistically significant. There is evidence from the rat bladder that in certain pathological conditions, such as inflammation (interstitial cystitis), P2X3 receptors become sensitised and there is also a prominent increase in the number of P2X3 receptor-positive neurons in pelvic and lumbar splanchnic pathways, perhaps contributing to the hypersensitivity seen in pathophysiological conditions [22]. Evidence for sensitization of P2X3 receptors on sensory nerves of the human ureter remains to be investigated.
The importance of purinergic mechanosensory transduction in the experience of ureteric pain in humans will ultimately be determined by clinical trials of pharmacological agents in patients with renal colic. We await the development of a potent, selective P2X3 receptor antagonist that is effective in vivo [23].
Abbreviations
- ABC:
-
avidin-biotin complex technique
- FITC:
-
fluorescein isothiocyanate
- NHS:
-
normal horse serum
- PBS:
-
phosphate-buffered saline
- TRPV1:
-
transient receptor potential vanilloid 1
References
Risholm L (1954) Studies on renal colic and its treatment by posterior splanchnic block. Acta Chir Scand Suppl 184:5–64
Amann R, Dray A, Hankins MW (1988) Stimulation of afferent fibres of the guinea-pig ureter evokes potentials in inferior mesenteric ganglion neurones. J Physiol 402:543–553
Edyvane KA, Smet PJ, Trussell DC et al (1994) Patterns of neuronal colocalisation of tyrosine hydroxylase, neuropeptide Y, vasoactive intestinal polypeptide, calcitonin gene-related peptide and substance P in human ureter. J Auton Nerv Syst 48:241–255. doi:10.1016/0165-1838(94)90053-1
Roza C, Laird JM, Cervero F (1998) Spinal mechanisms underlying persistent pain and referred hyperalgesia in rats with an experimental ureteric stone. J Neurophysiol 79:1603–1612
Ammons WS (1989) Primate spinothalamic cell responses to ureteral occlusion. Brain Res 496:124–130. doi:10.1016/0006-8993(89)91058-5
Sann H, Jancso G, Ambrus A et al (1995) Capsaicin treatment induces selective sensory degeneration and increased sympathetic innervation in the rat ureter. Neuroscience 67:953–966. doi:10.1016/0306-4522(95)00102-O
Schulman CC (1981) Innervation of the ureter. A histochemical and ultrastructural study. Anat Clin 3:127–142. doi:10.1007/BF01654504
Burnstock G (1999) Release of vasoactive substances from endothelial cells by shear stress and purinergic mechanosensory transduction. J Anat 194:335–342. doi:10.1046/j.1469-7580.1999.19430335.x
Ferguson DR, Kennedy I, Burton TJ (1997) ATP is released from rabbit urinary bladder epithelial cells by hydrostatic pressure changes—a possible sensory mechanism? J Physiol 505:503–511. doi:10.1111/j.1469-7793.1997.503bb.x
Burnstock G (2001) Purine-mediated signalling in pain and visceral perception. Trends Pharmacol Sci 22:182–188. doi:10.1016/S0165-6147(00)01643-6
Lee H-Y, Bardini M, Burnstock G (2000) Distribution of P2X receptors in the urinary bladder and ureter of the rat. J Urol 163:2002–2007. doi:10.1016/S0022-5347(05)67618-5
Namasivayam S, Eardley I, Morrison JF (1999) Purinergic sensory neurotransmission in the urinary bladder: an in vitro study in the rat. BJU Int 84:854–860. doi:10.1046/j.1464-410x.1999.00310.x
Vlaskovska M, Kasakov L, Rong W et al (2001) P2X3 knock-out mice reveal a major sensory role for urothelially released ATP. J Neurosci 21:5670–5677
Cockayne DA, Hamilton SG, Zhu Q-M et al (2000) Urinary bladder hyporeflexia and reduced pain-related behaviour in P2X3-deficient mice. Nature 407:1011–1015. doi:10.1038/35039519
Rong W, Burnstock G (2004) Activation of ureter nociceptors by exogenous and endogenous ATP in guinea pig. Neuropharmacology 47:1093–1101
Knight GE, Bodin P, de Groat WC et al (2002) ATP is released from guinea pig ureter epithelium on distension. Am J Physiol Renal Physiol 282:F281–F288
Hernández M, Barahona MV, Bustamante S et al (1999) A2B adenosine receptors mediate relaxation of the pig intravesical ureter: adenosine modulation of non adrenergic non cholinergic excitatory neurotransmission. Br J Pharmacol 126:969–978. doi:10.1038/sj.bjp.0702386
Cecchelli R, Cacan R, Porchet-Hennere E et al (1986) Dilatation of Golgi vesicles by monensin leads to enhanced accumulation of sugar nucleotides. Biosci Rep 6:227–234. doi:10.1007/BF01115011
Fujiwara T, Oda K, Yokota S et al (1988) Brefeldin A causes disassembly of the Golgi complex and accumulation of secretory proteins in the endoplasmic reticulum. J Biol Chem 263:18545–18552
Beacham WS, Kunze DL (1969) Renal receptors evoking a spinal vasometer reflex. J Physiol 201:73–85
Sann H, Cervero F (1988) Afferent innervation of the guinea-pig’s ureter. Agents Actions 25:243–245. doi:10.1007/BF01965024
Dang K, Lamb K, Cohen M et al (2008) Cyclophosphamide-induced bladder inflammation sensitizes and enhances P2X receptor function in rat bladder sensory neurons. J Neurophysiol 99:49–59. doi:10.1152/jn.00211.2007
Gever J, Cockayne DA, Dillon MP et al (2006) Pharmacology of P2X channels. Pflugers Arch 452:513–537. doi:10.1007/s00424-006-0070-9
Acknowledgements
The authors thank Dr Gillian E. Knight for editorial assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License ( https://creativecommons.org/licenses/by-nc/2.0 ), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Calvert, R.C., Thompson, C.S. & Burnstock, G. ATP release from the human ureter on distension and P2X3 receptor expression on suburothelial sensory nerves. Purinergic Signalling 4, 377–381 (2008). https://doi.org/10.1007/s11302-008-9123-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11302-008-9123-1