Abstract
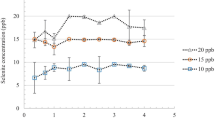
Several approaches have been used to estimate the bioaccessibility of trace metals from soils. Here, we applied phosphoric acid extraction and the in vitro test physiologically based extraction (PBET) to soils containing selenium (Se) and compared their performance in estimating the bioaccessibility of Se. For this purpose, we used two soil samples and two Certified Reference Material soil samples with a range of Se concentrations. The total Se contents were measured in the samples and in the extracts by hydride generation–atomic fluorescence spectroscopy. Moreover, we also measured selenite and selenate in the soil extracts (from phosphoric acid and from the PBET) using the coupled techniques liquid chromatography–UV photooxidation–atomic fluorescence spectroscopy and liquid chromatography–mass spectrometry with inductively coupled plasma. From the results obtained in the present study, the PBET showed that the selenium bioaccessible fraction was mainly attributed to the gastrointestinal step. When comparing the results from PBET with those of the phosphoric acid extraction, similar values of Se (IV) and Se (VI) were obtained for both extraction systems. An estimation of the bioaccessibility percentage of Se is also reported.


Similar content being viewed by others
References
Body, H. B., Pedersen, F., Cohr, K. H., Damborg, A., & Jakobser, B. M. (1999). Exposure scenarios and guidance values for urban soil pollutants. Regulatory Toxicology Pharmacology, 30, 197–208.
Bosso, S. T., & Enzweiler, J. (2008). Bioaccessible lead in soils, slag, and mine wastes from an abandoned mining district in Brazil. Environmental Geochemistry and Health, 30, 219–229.
Canadian Soil Quality Guidelines (2009). Selenium. Environmental and human health effects. CCME (Canadian Council of Ministers of Environment). ISBN:978-1-896997-90-2 PDF.
Carrizales, L., Razo, I., Téllez-Hernández, J. I., Torres-Nerio, R., Torres, A., Batres, L. E., et al. (2006). Exposure to arsenic and lead of children living near a copper-smelter in San Luis Potosi, Mexico: importance of soil contamination for exposure of children. Environmental Research, 101, 1–10.
Denys, S., Tack, K., Caboche, J., & Delalain, P. (2008). Bioaccessibility, solid phase distribution, and speciation of Sb in soils and in digestive fluids. Chemosphere, 74, 711–716.
Dhillon, K. S., & Dhillon, S. K. (2003). Distribution and management of seleniferous soils. Advances in Agronomy, 79, 119–184.
Dhillon, K. S., Dhillon, S. K., & Dogra, R. (2010). Selenium accumulation by forage and grain crops and volatilization from seleniferous soils amended with different organic materials. Chemosphere, 78, 548–556.
Drexler, J. W., & Brattin, W. J. (2007). An in vitro procedure for estimation of lead relative bioavailability: with validation. Human and Ecological Risk Assessment, 13, 383–401.
Environment Agency U.K. (2009). Soil guideline values for selenium in soil. Science report SC050021/Selenium SGV.
Environmental Protection, England and Wales (2005). The landfill (England and Wales) (Amendment) Regulations. No. 1640. Available at: http://www.opsi.gov.uk/si/si2005/20051640.htm. Accessed 5 Apr 2011.
Fessler, A. J., Moller, G., Talcott, P. A., & Exon, J. H. (2003). Selenium toxicity in sheep grazing reclaimed phosphate mining sites. Veterinary and Human Toxicology, 45, 294–298.
Finley, J. W. (2007). Increased intakes of selenium-enriched foods may benefit human health. Review. Journal of the Science of Food and Agriculture, 87, 1620–1629.
Goldberg, S., Martens, D. A., Forster, H. S., & Herbel, M. J. (2006). Speciation of selenium (IV) and selenium (VI) using coupled ion chromatography-hydride generation atomic absorption spectrometry. Soil Science Society of American Journal, 70, 41–47.
González-Nieto, J., López-Sánchez, J. F., & Rubio, R. (2006). Comparison of chemical modifiers for selenium determination in soil aqua regia extracts by ZETAAS. Talanta, 69, 1118–1122.
Hansen, J. B., Oomen, A. G., Edelgaard, I., & Grøn, C. (2007). Oral bioaccessibility and leaching: tests for soil risk assessment. Engineering in Life Sciences, 7, 170–176.
Hartikainen, H. (2005). Biogeochemistry of selenium and its impact on food chain quality and human health. Journal of Trace Elements in Medicine and Biology, 18, 309–318.
Hawkesford, M. J., & Zhao, F. L. (2006). Strategies for increasing the selenium content in wheat. Journal of Cereal Science, 46, 282–292.
Intawongse, M., & Dean, J. R. (2006). In vitro testing for assessing oral bioaccessibility of trace metals in soil and food samples. TrAC. Trends in Analytical Chemistry, 25, 876–886.
ISO 11466. (1995). Soil quality-extraction of trace elements soluble in aqua regia. International organization for standardization. Geneva. Switzerland.
Kabata-Pendias, A., & Pendias, H. (2001). Trace elements in soils and plants (3rd ed.). Boca Raton: CRC Press.
Keskinen, R., Ekholm, P., Yli-Halla, M., & Hartikainen, H. (2009). Efficiency of different methods in extracting selenium from agricultural soils of Finland. Geoderma, 153, 87–93.
Latawiec, A. E., Simmons, P., & Reid, B. J. (2010). Decision-makers’ perspectives on the use of bioaccessibility for risk-based regulation of contaminated land. Environment International, 36, 383–389.
Lemly, A. D. (2007). A procedure for NEPA assessment of selenium hazards associated with mining. Environmental Monitoring and Assessment, 125, 361–375.
Ljung, K., Oomen, A., Duits, M., Selinus, O., & Berglund, M. (2007). Bioaccessibility of metals in urban playground soils. Journal of Environmental Science and Health. Part A: Toxic/Hazardous Substances and Environmental Engineering, 42, 1241–1250.
Madrid, F., Biasioli, M., & Ajmone-Marsan, F. (2008). Availability and bioaccessibility of metals in fine particles of some urban soils. Archives of Environmental Contamination and Toxicology, 55, 21–32.
Makris, K. C., Quazi, S., Nahas, R., Sarkar, D., Datta, R., & Sylvia, V. L. (2008). In vitro model improves the prediction of soil arsenic bioavailability: worst-case scenario. Environmental Science & Technology, 42, 6278–6284.
Meunier, L., Wragg, J., Koch, I., & Reimer, K. J. (2010). Method variables affecting the bioaccessibility of arsenic in soil. Journal of Environmental Science and Health. Part A: Toxic/Hazardous Substances and Environmental Engineering, 45, 517–526.
Morman, S. A., Plumlee, G. S., & Smith, D. B. (2009). Application of in vitro extraction studies to evaluate element bioaccessibility in soils from a transect across the United States and Canada. Applied Geochemistry, 4, 1454–1463.
Orero Iserte, L., Roig-Navarro, A. F., & Hernéndez, F. (2004). Simultaneous determination of arsenic and selenium species in phosphoric acid extracts of sediment samples by HPLC-ICP-MS. Analytica Chimica Acta, 527, 97–104.
Pouschat, P., & Zagury, G. J. (2006). In vitro gastrointestinal bioavailability of arsenic in soils collected near CCA-treated utility poles. Environmental Science & Technology, 40, 4317–4323.
Reeder, R. J., Schoonen, M. A. A., & Lanzirotti, A. (2006). Metal speciation and its role in bioaccessibility and bioavailability. Reviews in Mineralogy and Geochemistry, 64, 59–113.
Ruby, M. V., Davis, A., Schoof, R., Eberle, S., & Sellstone, C. M. (1996). Estimation of lead and arsenic bioavailability physiologically based extraction test. Environmental Science & Technology, 30, 422–430.
Ruiz-Chancho, M. J., López-Sánchez, J. F., & Rubio, R. (2007). Analytical speciation as a tool to assess arsenic behaviour in soils polluted by mining. Analytical and Bioanalytical Chemistry, 387, 627–635.
Scientific Committee on Food (2000). SCF/CS/NUT/UPPLEV/25 Final. Opinion of the scientific committee of food on the tolerable upper intake level of selenium. European Comission. Health & Consumer Protection Directorate General. European Commission. Brussels. Belgium
Sharma, S., Vance, G. F. (2007) Dissolution chemistry of inorganic selenium in alkaline mine. In D. Sarkar, R. Datta and R. Hannigan. (Eds.), Developments in environmental sciences Vol 5. Oxford: Elsevier.
Smith, B. A., Kirk, J. L., & Stephenson, G. L. (2010). The influence of liquid to soil ratios on arsenic and lead bioaccessibility in reference and field soil. Human and Ecological Risk Assessment, 16, 149–162.
Smith, B., Rawlins, B. G., Cordeiro, M. J. A. R., Hutchins, M. G., Tiberindwa, J. V., Sserinjogi, L., et al. (2000). The bioaccessibility of essential and potentially toxic trace elements in tropical soils from Mukono District, Uganda. Journal of the Geological Society, 157, 885–891.
Smith, E., Weber, J., & Juhasz, A. L. (2009). Arsenic distribution and bioaccessibility across particle fractions in historically contaminated soils. Environmental Geochemistry and Health, 31, 85–92.
Somogyi, Z., Kiss, I., Kádár, I., & Bakoni, G. (2007). Toxicity of selenate and selenite to the potworm Enchytraeus albidus (Annelida: Enchytraeidae): a laboratory test. Ecotoxicology, 16, 379–384.
Spadoni, M., Voltaggio, M., Carcea, M., Coni, E., Raggi, A., & Cubadda, F. (2007). Bioaccessible selenium in Italian agricultural soils: comparison of the biogeochemical and pedoclimatic variables. The Science of the Total Environment, 376, 160–177.
Uden, P. C. (2005). Speciation of Selenium. In R. Cornelis, J. Caruso, H. Crews & K. Heumann. (Eds.), Handbook of Elemental Speciation II—Species in the Environment, Food, Medicine and Occupational Health. Chichester: Wiley
US EPA (2009). Highlights of the child-specific exposure factors handbook. National Center for Environmental Assessment. Washington, DC; EPA/600/R-08/135. Available on: http://www.epa.gov/ncea. Accessed 5 Apr 2011.
Wragg, J. J., Cave, M. R., & for the Environmental Agency. (2002). In vitro methods for the measurement of the oral bioaccessibility of selected metals and metalloids in soils: A critical review. R&D technical report P5-062/TR/01. Environmental Agency. Bristol. UK.
Zagury, G. J., Bedeaux, C., & Welfringer, B. (2009). Influence of mercury speciation and fractionation on bioaccessibility in soils. Archives of Environmental Contamination and Toxicology, 56, 371–379.
Acknowledgments
The authors are grateful to the Dirección General de Investigación (DGICyT) for Project number CTQ2010-15377/BQU and the Departament d’Universitats, Recerca i Societat de la Informació for financial support (SGR2009-1188). Virginia Funes thanks the University of Barcelona for a predoctoral grant. The authors also thank Dr. Toni Padró from the Serveis Científico-Tècnics of the University of Barcelona for his valuable help in the ICPMS measurements. The authors thank Dr. Zhi-Qing Lin for supplying R1 and R3 soil samples.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Funes-Collado, V., Rubio, R. & López-Sánchez, J.F. Comparison of In Vitro PBET and Phosphoric Acid Extraction as an Approach to Estimate Selenite and Selenate Bioaccessibility from Soil. Water Air Soil Pollut 222, 315–324 (2011). https://doi.org/10.1007/s11270-011-0826-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11270-011-0826-5