Abstract
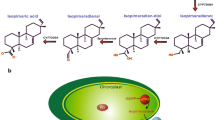
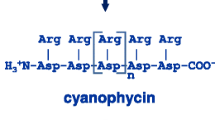
Increasing the arginine (Arg) content in plants used as feed or food is of interest, since the supplementation of food with conditionally essential Arg has been shown to have nutritional benefits. An increase was achieved by the expression of the Arg-rich bacterial storage component, cyanophycin (CGP), in the chloroplast of transgenic plants. CGP is stable in plants and its degradation into β-aspartic acid (Asp)-Arg dipeptides, is solely catalyzed by bacterial cyanophycinases (CGPase). Dipeptides can be absorbed by animals even more efficiently than free amino acids (Matthews and Adibi 1976; Wenzel et al. 2001). The simultaneous production of CGP and CGPase in plants could be a source of β-Asp-Arg dipeptides if CGP degradation can be prevented in planta or if dipeptides are stable in the plants. We have shown for the first time that it is possible to co-express CGP and CGPase in the same plant without substrate degradation in planta by transient expression of the cyanobacterial CGPase CPHB (either in the plastid or cytosol), and the non-cyanobacterial CGPase CPHE (cytosol) in CGP-producing Nicotiana tabacum plants. We compared their ability to degrade CGP in planta and in crude plant extracts. No CGP degradation appeared prior to cell homogenization independent of the CGPase produced. In crude plant extracts, only cytosolic CPHE led to a fast degradation of CGP. CPHE also showed higher stability and in vitro activity compared to both CPHB variants. This work is the next step to increase Arg in forage plants using a stable, Arg-rich storage protein.



Similar content being viewed by others
References
Abad MS, Clark SE, Lamppa GK (1989) Properties of a chloroplast enzyme that cleaves the chlorophyll a/B binding-protein precursor—optimization of an organelle-free reaction. Plant Physiol 90:117–124. doi:10.1104/Pp.90.1.117
Allen MM, Morris R, Zimmerman W (1984) Cyanophycin granule polypeptide protease in a unicellular cyanobacterium. Arch Microbiol 138:119–123
Bröer S (2008) Amino acid transport across mammalian intestinal and renal epithelia. Physiol Rev 88:249–286
Gleba Y, Klimyuk V, Marillonnet S (2005) Magnifection—a new platform for expressing recombinant vaccines in plants. Vaccine 23:2042–2048. doi:10.1016/j.vaccine.2005.01.006
Gupta M, Carr NG (1981) Enzyme-activities related to cyanophycin metabolism in heterocysts and vegetative cells of Anabaena spp. J Gen Microbiol 125:17–23
Hu SD, Li XL, Rezaei R, Meininger CJ, McNeal CJ, Wu GY (2015) Safety of long-term dietary supplementation with largening in pigs. Amino Acids 47:925–936. doi:10.1007/s00726-015-1921-5
Hühns M et al (2008) Plastid targeting strategies for cyanophycin synthetase to achieve high-level polymer accumulation in Nicotiana tabacum. Plant Biotechnol J 6:321–336. doi:10.1111/j.1467-7652.2007.00320.x
Hühns M et al (2009) Tuber-specific cphA expression to enhance cyanophycin production in potatoes. Plant Biotechnol J 7:883–898. doi:10.1111/j.1467-7652.2009.00451.x
Lamppa GK, Abad MS (1987) Processing of a wheat light-harvesting chlorophyll a/B protein-precursor by a soluble enzyme from higher-plant chloroplasts. J Cell Biol 105:2641–2648. doi:10.1083/jcb.105.6.2641
Ma X, Zheng C, Hu Y, Wang L, Yang X, Jiang Z (2015) Dietary l-arginine supplementation affects the skeletal longissimus muscle proteome in finishing pigs. PLoS ONE 10:1–16
Marillonnet S, Thoeringer C, Kandzia R, Klimyuk V, Gleba Y (2005) Systemic agrobacterium tumefaciens-mediated transfection of viral replicons for efficient transient expression in plants. Nat Biotechnol 23:718–723. doi:10.1038/Nbt1094
Matthews DM, Adibi SA (1976) Peptide absorption. Gastroenterology 71:151–161
Nausch H, Broer I (2016) Cyanophycinase CphE from P. alcaligenes produced in different compartments of N. benthamiana degrades high amounts of cyanophycin in plant extracts. Appl Microbiol Biotechnol. doi:10.1007/s00253-016-8020-8
Nausch H, Mikschofsky H, Koslowski R, Meyer U, Broer I, Huckauf J (2012a) High-level transient expression of ER-targeted human interleukin 6 in Nicotiana benthamiana. PLoS ONE. doi:10.1371/journal.pone.0048938
Nausch H, Mischofsky H, Koslowski R, Meyer U, Broer I, Huckauf J (2012b) Expression and subcellular targeting of human complement factor C5a in Nicotiana species. PLoS ONE. doi:10.1371/journal.pone.0053023
Nausch H et al (2016) Tobacco as platform for a commercial production of cyanophycin. New Biotechnol. doi:10.1016/j.nbt.2016.08.001
Neubauer K et al (2012) Isolation of cyanophycin from tobacco and potato plants with constitutive plastidic cphA(Te) gene expression. J Biotechnol 158:50–58. doi:10.1016/j.jbiotec.2011.12.008
Neumann K, Stephan DP, Ziegler K, Huhns M, Broer I, Lockau W, Pistorius EK (2005) Production of cyanophycin, a suitable source for the biodegradable polymer polyaspartate, in transgenic plants. Plant Biotechnol J 3:249–258. doi:10.1111/j.1467-7652.2005.00122.x
Obst M, Oppermann-Sanio FB, Luftmann H, Steinbuchel A (2002) Isolation of cyanophycin-degrading bacteria, cloning and characterization of an extracellular cyanophycinase gene (cphE) from Pseudomonas anguilliseptica strain BI—the cphE gene from P. anguilliseptica BI encodes a cyanophycin-hydrolyzing enzyme. J Biol Chem 277:25096–25105. doi:10.1074/jbc.M112267200
Pillay P, Schluter U, van Wyk S, Kunert KJ, Vorster BJ (2014) Proteolysis of recombinant proteins in bioengineered plant cells. Bioengineered 5:15–20. doi:10.4161/Bioe.25158
Ponndorf D et al (2016) Stable production of cyanophycinase in Nicotiana benthamiana and its functionality to hydrolyse cyanophycin in the murine intestine. Plant Biotechnol J. doi:10.1111/pbi.12658
Richter S, Lamppa GK (1998) A chloroplast processing enzyme functions as the general stromal processing peptidase. Proc Natl Acad Sci USA 95:7463–7468
Richter R, Hejazi M, Kraft R, Ziegler K, Lockau W (1999) Cyanophycinase, a peptidase degrading the cyanobacterial reserve material multi-L-arginyl-poly-l-aspartic acid (cyanophycin)—molecular cloning of the gene of Synechocystis sp. PCC 6803, expression in Escherichia coli, and biochemical characterization of the purified enzyme. Eur J Biochem 263:163–169. doi:10.1046/j.1432-1327.1999.00479.x
Robinson C, Ellis RJ (1984) Transport of proteins into chloroplasts. Partial purification of a chloroplast protease involved in the processing of important precursor polypeptides. Eur J Biochem 142:337–342
Sallam A, Steinbuchel A (2009a) Cyanophycin-degrading bacteria in digestive tracts of mammals, birds and fish and consequences for possible applications of cyanophycin and its dipeptides in nutrition and therapy. J Appl Microbiol 107:474–484. doi:10.1111/j.1365-2672.2009.04221.x
Sallam A, Steinbuchel A (2009b) Process for the preparation of dipeptides from cyanophycin emplyoing the isolated Pseudomonas alcaligenes DIP1 CGPase CphEaI. European Patent Application, Publication No. 2 133 419 A1 (16.12.2009)
Sallam A, Steinbuchel A (2010) Dipeptides in nutrition and therapy: cyanophycin-derived dipeptides as natural alternatives and their biotechnological production. Appl Microbiol Biotechnol 87:815–828. doi:10.1007/s00253-010-2641-0
Sallam A, Kast A, Przybilla S, Meiswinkel T, Steinbuchel A (2009) Biotechnological process for production of beta-dipeptides from cyanophycin on a technical scale and its optimization. Appl Environ Microbiol 75:29–38. doi:10.1128/AEM.01344-08
Sallam A, Kalkandzhiev D, Steinbuchel A (2011) Production optimization of cyanophycinase ChpEal from Pseudomonas alcaligenes DIP1. AMB Express 1:38. doi:10.1186/2191-0855-1-38
Sancho-Vaello E, Fernandez-Murga ML, Rubio V (2009) Mechanism of arginine regulation of acetylglutamate synthase, the first enzyme of arginine synthesis. FEBS Lett 583:202–206. doi:10.1016/j.febslet.2008.12.001
Sheen J, Hwang SB, Niwa Y, Kobayashi H, Galbraith DW (1995) Green-fluorescent protein as a new vital marker in plant-cells. Plant J 8:777–784. doi:10.1046/j.1365-313X.1995.08050777.x
Simon RD (1987) Inclusion bodies in the cyanobacteria: cyanophycin, polyphosphate, polyhedra bodies. In: Fay P, Van Baalen C (eds) The cyanobacteria. Elsevier, Amsterdam, pp 192–222
Simon RD, Weathers P (1976) Determination of structure of novel polypeptide containing aspartic-acid and arginine which is found in cyanobacteria. Biochem Biophys Acta 420:165–176
Stothard P (2000) The sequence manipulation suite: JavaScript programs for analyzing and formatting protein and DNA sequences. Biotechniques 28:1102
Utagawa T (2004) Production of arginine by fermentation. J Nutr 134:2854s–2857s
Wang B et al (2015) Effects of dietary arginine supplementation on growth performance, flesh quality, muscle antioxidant capacity and antioxidant-related signalling molecule expression in young grass carp (Ctenopharyngodon idella). Food Chem 167:91–99. doi:10.1016/j.foodchem.2014.06.091
Wenzel U, Meissner B, Doring F, Daniel H (2001) PEPT1-mediated uptake of dipeptides enhances the intestinal absorption of amino acids via transport system b(0, +). J Cell Physiol 186:251–259. doi:10.1002/1097-4652(200102)186:2<251:AID-JCP1027>3.0.CO;2-F
Winter G, Todd CD, Trovato M, Forlani G, Funck D (2015) Physiological implications of arginine metabolism in plants. Front Plant Sci. doi:10.3389/Fpls.2015.00534
Wu G et al (2007) Pharmacokinetics and safety of arginine supplementation in animals. J Nutr 137:1673S–1680S
Wu GY, Bazer FW, Dai ZL, Li DF, Wang JJ, Wu ZL (2014) Acid nutrition in animals: protein synthesis and beyond amino. Annu Rev Anim Biosci 2:387–417. doi:10.1146/annurev-animal-022513-114113
Ziegler K, Diener A, Herpin C, Richter R, Deutzmann R, Lockau W (1998) Molecular characterization of cyanophycin synthetase, the enzyme catalyzing the biosynthesis of the cyanobacterial reserve material multi-L-arginyl-poly-l-aspartate (cyanophycin). Eur J Biochem 254:154–159
Ziegler K, Deutzmann R, Lockau W (2002) Cyanophycin synthetase-like enzymes of non-cyanobacterial eubacteria: characterization of the polymer produced by a recombinant synthetase of Desulfitobacterium hafniense Zeitschrift Fur Naturforschung section C-a. J Biosci 57:522–529
Acknowledgements
We would like to express our very great appreciations to Alex Rajewski for proof-reading and the helpful suggestions and Dr. Martin Krehenbrink, Cysal GmbH, for sharing their aquaculture results.
Funding
This study was funded by the University of Rostock.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Ponndorf, D., Broer, I. & Nausch, H. Expression of CphB- and CphE-type cyanophycinases in cyanophycin-producing tobacco and comparison of their ability to degrade cyanophycin in plant and plant extracts. Transgenic Res 26, 491–499 (2017). https://doi.org/10.1007/s11248-017-0019-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11248-017-0019-0