Abstract
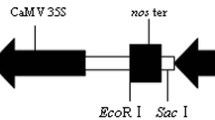
Sugarcane is a prime crop for commercial production of bioethanol and table sugar. Genetic engineering is complementing traditional breeding in sugarcane improvement. Costs and delays associated with regulatory approval for a transgenic event are substantial impediments to the commercialization of transgenic crops. Generation of intragenic sugarcane events carrying additional sequences from sexually compatible sorghum resembles traditional plant breeding and may facilitate regulatory approval. Acetolactate synthase (ALS), is a key enzyme in the biosynthetic pathway of branched-chain amino acids, and also a target for ALS-inhibiting herbicides including sulfonylureas and imidazolinones. In this study, we developed an intragenic minimal expression cassette encoding a mutant sorghum acetolactate synthase gene (mALS) under transcriptional control of the sorghum ubiquitin promoter and sorghum HSP 3′UTR. mALS was delivered into tissue cultures of sugarcane cultivar CP 88-1762 by biolistic gene transfer. A suitable selection protocol for recovery of intragenic events was developed for the herbicide bispyribac sodium. Intragenic sugarcane events were identified by PCR, and qRT-PCR analysis revealed events with different levels of mALS expression. Expression of the mALS gene in sugarcane resulted in chlorsulfuron resistance, as demonstrated by lower injury level and increased plant height following application of chlorsulfuron compared to the control cultivar. The results demonstrated that the mALS gene from sorghum supports the production of intragenic, herbicide resistant sugarcane. This is the first report of an intragenic selectable marker for sugarcane.




Similar content being viewed by others
References
Altpeter F, Oraby H (2010) Sugarcane. In: Kempken F, Jung C (eds) Genetic modification of plants. Springer, Berlin, pp 453–472
Altpeter F, Sandhu S (2010) Genetic transformation–biolistics. In: Davey MR, Anthony P (eds) Plant cell culture: essential methods. Scion Publishing Ltd, Oxfordshire, pp 217–240
Arencibia AD, Carmona ER, Tellez P, Chan MT, Yu SM, Trujillo LE, Oramas P (1998) An efficient protocol for sugarcane (Saccharum spp. L.) transformation mediated by Agrobacterium tumefaciens. Transgenic Res 7:213–222
Bower R, Birch RG (1992) Transgenic sugarcane plants via microprojectile bombardment. Plant J 2:409–416
Bower R, Elliott AR, Potier BAM, Birch RG (1996) High-efficiency, microprojectile-mediated cotransformation of sugarcane, using visible or selectable markers. Mol Breed 2:239–249
Bradford KJ, Van Deynze A, Gutterson N, Parrott W, Strauss SH (2005) Regulating transgenic crops sensibly: lessons from plant breeding, biotechnology and genomics. Nat Biotechnol 23:439–444
Brown HM (1990) Mode of action, crop selectivity, and soil relations of the sulfonylurea herbicides. Pestic Sci 29:263–281
Cheavegatti-Gianotto A, de Abreu HM, Arruda P, Bespalhok Filho JC, Burnquist WL, Creste S, di Ciero L, Ferro JA, de Oliveira Figueira AV, de Sousa Filgueiras T, Grossi-de-Sá MD, Guzzo EC, Hoffmann HP, de Andrade Landell MG, Macedo N, Matsuoka S, de Castro Reinach F, Romano E, da Silva WJ, de Castro Silva Filho M, César Ulian E (2011) Sugarcane (Saccharum × officinarum): a reference study for the regulation of genetically modified cultivars in Brazil. Trop Plant Biol 4:62–89
Chengalrayan K, Gallo-Meagher M (2001) Effect of various growth regulators on shoot regeneration of sugarcane. In Vitro Cell Dev Biol Plant 37:434–439
Christensen AH, Quail PH (1996) Ubiquitin promoter-based vectors for high-level expression of selectable and/or screenable marker genes in monocotyledonous plants. Transgenic Res 5:213–218
Endo M, Shimizu T, Toki S (2012) Selection of transgenic rice plants using a herbicide tolerant form of the acetolactate synthase gene. Methods Mol Biol 847:59–66
Holme IB, Wendt T, Holm PB (2013) Intragenesis and cisgenesis as alternatives to transgenic crop development. Plant Biotechnol J 11:395–407
Iskandar HM, Simpson RS, Casu RE, Bonnett GD, Maclean DJ, Manners JM (2004) Comparison of reference genes for quantitative real-time polymerase chain reaction analysis of gene expression in sugarcane. Plant Mol Biol Rep 22:325–337
Joyce P, Kuwahata M, Turner N, Lakshmanan P (2010) Selection system and co-cultivation medium are important determinants of Agrobacterium-mediated transformation of sugarcane. Plant Cell Rep 29:173–183
Jung JH, Fouad WM, Vermerris W, Gallo M, Altpeter F (2012) RNAi suppression of lignin biosynthesis in sugarcane reduces recalcitrance for biofuel production from lignocellulosic biomass. Plant Biotechnol J 10:1067–1076
Jung JH, Vermerris W, Gallo M, Fedenko JR, Erickson JE, Altpeter F (2013) RNA interference suppression of lignin biosynthesis increases fermentable sugar yields for biofuel production from field-grown sugarcane. Plant Biotechnol J 11:709–716
Jung JH, Kannan B, Dermawan H, Moxley GW, Altpeter F (2016) Precision breeding for RNAi suppression of a major 4-coumarate:coenzyme A ligase gene improves cell wall saccharification from field grown sugarcane (under review for publication)
Kawai K, Kaku K, Izawa N, Shimizu T, Fukuda A, Tanaka Y (2007a) A novel mutant acetolactate synthase gene from rice cells, which confers resistance to ALS-inhibiting herbicides. J Pestic Sci 32:89–98
Kawai K, Kaku K, Izawa N, Fukuda A, Tanaka Y, Shimizu T (2007b) Functional analysis of transgenic rice plants expressing a novel mutated ALS gene of rice. J Pestic Sci 32:385–392
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods 25:402–408
Lusser M, Parisi C, Plan D, Rodríguez-Cerezo E (2012) Deployment of new biotechnologies in plant breeding. Nat Biotechnol 30:231–239
Mudge SR, Basnayake SW, Moyle RL, Osabe K, Graham MW, Morgan TE, Birch RG (2013) Mature-stem expression of a silencing-resistant sucrose isomerase gene drives isomaltulose accumulation to high levels in sugarcane. Plant Biotechnol J 1:502–509
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:473–497
Murray MG, Thompson WF (1980) Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res 8:4321–4326
Nagaya S, Kawamura K, Shinmyo A, Kato K (2010) The HSP terminator of Arabidopsis thaliana increases gene expression in plant cells. Plant Cell Physiol 51:328–332
Ogawa T, Kawahigashi H, Toki S, Handa H (2008) Efficient transformation of wheat by using a mutated rice acetolactate synthase gene as a selectable marker. Plant Cell Rep 27:1325–1331
Osakabe K, Endo M, Kawai K, Nishizawa Y, Ono K, Abe K, Ishikawa Y, Nakamura H, Ichikawa H, Nishimura S, Shimizu T, Toki S (2005) The mutant form of acetolactate synthase genomic DNA from rice is an efficient selectable marker for genetic transformation. Mol Breed 16:313–320
Powles SB, Yu Q (2010) Evolution in action: plants resistant to herbicides. Annu Rev Plant Biol 61:317–347
Punyadee P, Thongros M, Pornprom T (2007) Biochemical mechanism of resistance to imazapyr in sugarcane cell selections. Thai J Agric Sci 40:133–141
Rainbolt C, Dusky J (2006) Weed management in sugarcane. 1:1–8
Rommens CM (2004) All-native DNA transformation: a new approach to plant genetic engineering. Trends Plant Sci 9:457–464
Rommens CM, Haring MA, Swords K, Davies HV, Belknap WR (2007) The intragenic approach as a new extension to traditional plant breeding. Trends Plant Sci 12:397–403
Saari LL, Cotterman JC, Thill DC (1994) Resistance to acetolactate synthase inhibiting herbicides. In: Poweles SB, Holtum JAM (eds) Herbicide resistance in plants: biology and biochemistry. Ann Arbor, Lewis, pp 83–139
Schouten HJ, Krens FA, Jacobsen E (2006) Cisgenic plants are similar to traditionally bred plants: international regulations for genetically modified organisms should be altered to exempt cisgenesis. EMBO Rep 7:750–753
Taniguchi Y, Kawata M, Ando I, Shimizu T, Ohshima M (2010) Selecting genetic transformants of indica and indica-derived rice cultivars using bispyribac sodium and a mutated ALS gene. Plant Cell Rep 29:1287–1295
Taparia Y, Fouad WM, Gallo M, Altpeter F (2012a) Rapid production of transgenic sugarcane with the introduction of simple loci following biolistic transfer of a minimal expression cassette and direct embryogenesis. In Vitro Cell Dev Biol Plant 48:15–22
Taparia Y, Gallo M, Altpeter F (2012b) Comparison of direct and indirect embryogenesis protocols, biolistic gene transfer and selection parameters for efficient genetic transformation of sugarcane. Plant Cell, Tissue Organ Cult 111:131–141
Tranel PJ, Wright TR, Heap IM (2016) Mutations in herbicide-resistant weeds to ALS inhibitors. http://www.weedscience.com
Van der Vyver C, Conradie T, Kossmann J, Lloyd J (2013) In vitro selection of transgenic sugarcane callus utilizing a plant gene encoding a mutant form of acetolactate synthase. In Vitro Cell Dev Biol Plant 49:198–206
Zale J, Jung JH, Kim JY, Pathak B, Karan R, Liu H, Chen X, Wu H, Candreva J, Zhai Z, Shanklin J, Altpeter F (2016) Metabolic engineering of sugarcane to accumulate energy-dense triacylglycerols in vegetative biomass. Plant Biotechnol J 14:661–669
Zhangsun D, Luo S, Chen R, Tang K (2007) Improved Agrobacterium-mediated genetic transformation of GNA transgenic sugarcane. Biologia (Bratisl.) 62:386–393
Zhou M, Luo H (2013) MicroRNA-mediated gene regulation: potential applications for plant genetic engineering. Plant Mol Biol 83:59–75
Acknowledgments
The authors would like to thank Dr. Hardev Sandhu (Everglades Research and Educational Center, UF-IFAS, Belle Glade, FL) for providing tops of sugarcane cultivar CP 88-1762 and Sun Gro Horticulture, Apopka, FL for donation of the Fafard #2 potting mix.
Authors’ contributions
FA conceived the experiments. FA, RK and JHJ designed the experiments. JHJ constructed the plasmid. HD, RK, YZ, SP and GS generated the intragenic plants, RK, JHJ and HD analyzed the intragenic plants. RK, HD and FA wrote the manuscript. All authors read and approved the final manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Dermawan, H., Karan, R., Jung, J.H. et al. Development of an intragenic gene transfer and selection protocol for sugarcane resulting in resistance to acetolactate synthase-inhibiting herbicide. Plant Cell Tiss Organ Cult 126, 459–468 (2016). https://doi.org/10.1007/s11240-016-1014-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-016-1014-5