Abstract
Selection genes are routinely used in plant genetic transformation protocols to ensure the survival of transformed cells by limiting the regeneration of non-transgenic cells. In order to find alternatives to the use of antibiotics as selection agents, we followed a targeted approach utilizing a plant gene, encoding a mutant form of the enzyme acetolactate synthase, to convey resistance to herbicides. The sensitivity of sugarcane callus (Saccharum spp. hybrids, cv. NCo310) to a number of herbicides from the sulfonylurea and imidazolinone classes was tested. Callus growth was most affected by sulfonylurea herbicides, particularly 3.6 μg/l chlorsulfuron. Herbicide-resistant transgenic sugarcane plants containing mutant forms of a tobacco acetolactate synthase (als) gene were obtained following biolistic transformation. Post-bombardment, putative transgenic callus was selectively proliferated on MS medium containing 3 mg/l 2,4-dichlorophenoxyacetic acid (2,4-D), 20 g/l sucrose, 0.5 g/l casein, and 3.6 μg/l chlorsulfuron. Plant regeneration and rooting was done on MS medium lacking 2,4-D under similar selection conditions. Thirty vigorously growing putative transgenic plants were successfully ex vitro-acclimatized and established under glasshouse conditions. Glasshouse spraying of putative transgenic plants with 100 mg/l chlorsulfuron dramatically decreased the amount of non-transgenic plants that had escaped the in vitro selection regime. PCR analysis showed that six surviving plants were als-positive and that five of these expressed the mutant als gene. This report is the first to describe a selection system for sugarcane transformation that uses a selectable marker gene of plant origin targeted by a sulfonylurea herbicide.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since the early 1990s of the last century, much progress has been made in the biotechnological manipulation of sugarcane. Both in vitro regeneration and genetic transformation were established and are now routinely used (Franks and Birch 1991; Bower and Birch 1992). These technologies, however, depend heavily on the antibiotic selection of in vitro genetically transformed cells. In the past, antibiotic and herbicide systems that were predominantly used as selectable marker genes in the process of transgenic sugarcane production included the antibiotic nptII (neomycin phosphotransferase) and hpt (hygromycin phosphotransferase) genes, as well as the bar (phosphinotricin acetyltransferase) gene (Enriquez-Obregon et al. 1998; Leibbrandt and Snyman 2003; Joyce et al. 2010). To date, only one other selection system, namely, mannose in combination with the Escherichia coli phosphomannose isomerase gene, has been tested in sugarcane (Chengalrayan et al. 2001; Jain et al. 2007). However, the escape rate for this in vitro selection system was more than 40%, with 15 of 34 putative transgenic clones being false positives. Since the existing selection systems for use in sugarcane transformation are predominantly based on antibiotic resistance, there is an urgent need for the development of alternative selection systems that might be more acceptable to producers and consumers. Even though some of these antibiotic selectable markers have been declared safe to use in transgenic crops by the US Environmental Protection Agency (1994), they are still not acceptable to a large portion of consumers (Franks 1999; Chern et al. 2002). Developing additional alternative selection systems for specific crops will also allow for repeated transformations where more than one selection system is needed for multiple gene transfer in gene stacking approaches into a particular plant species.
In recent years, alternative selection systems have been developed, including the so-called positive selection systems that can be used to supply the putative transgenic plants cells with a metabolic advantage over non-transformed cells (Joersbo and Okkels 1996). In addition, plant genes containing amino acid substitutions leading to decreased herbicide binding have been used for selection (Li et al. 1992; Ogawa et al. 2008; Endo et al. 2012). One such plant gene is acetolactate synthase (ALS) targeted by a large number of herbicides including members of sulfonylurea, imidazolinone, triazolopyrimidine, pyrimidinyl-thiobenzoate, and sulfonyl-aminocarbonyl-triazolinone (LaRossa and Schloss 1984; Mazur and Falco 1989; Duggleby and Pang 2000). ALS is the first enzyme in the biosynthetic pathway for the branched-chain amino acids valine, leucine, and isoleucine (Chaleff and Mauvais 1984; Yadav et al. 1986; Singh and Shaner 1995). The enzyme is further nuclear-encoded and targeted to the chloroplast (Corbett and Tardif 2006). It catalyzes the condensation of two pyruvate molecules to acetolactate as well as the condensation of pyruvate and ketobutyrate to acetohyroxybutyrate (LaRossa and Schloss 1984; Schloss et al. 1985; Yadav et al. 1986; Singh and Shaner 1995). Herbicides act on the catalytic region of ALS as competitive inhibitors for pyruvate (Chaleff and Mauvais 1984; McCourt et al. 2006), resulting in the inactivation of the enzyme and the subsequent halting of branched amino acid synthesis that leads to plant death.
Mutations in the als gene of E. coli (Yadav et al. 1986), Saccharomyces cerevisiae (Falco and Dumas 1985; Yadav et al. 1986), and plants (Chaleff and Mauvais 1984; Chaleff and Ray 1984; Lee et al. 1988; Kochevenko and Willmitzer 2003; Khruangchan et al. 2011; Schnell et al. 2012) confer resistance to herbicides. Single nucleotide changes in the gene result in an amino acid substitution, rendering the enzyme less sensitive to inhibition. When reviewed in 2010, there were 22 substitutions at seven sites across als genes leading to resistance (Powles and Yu 2010). The most prominent mutation is Pro-197 in which 11 amino acid substitutions affect herbicide resistance in almost 50 plant species (Warwick et al. 2008). Pro-197 mutations result mainly in sulfonylurea resistance, whereas mutations at Ala-205, Asp-376, Trp-574, Ser-653, or Gly-654 cause resistance against imidazolinone and, to a lesser extent, sulfonylurea herbicides in plants such as canola, rice, sugar beet, maize, wheat, and cotton (Sebastian et al. 1989; Swanson et al. 1989; Newhouse et al. 1991, 1992; Rajasekaran et al. 1996; Wright and Penner 1998; Bae et al. 2002; Laplante et al. 2009). So far, only one mutation (Ala-559) in the als gene has been identified in field-grown sugarcane conferring tolerance to the herbicide imazapyr (Punyadee et al. 2007; Khruangchan et al. 2011).
A few of these als gene mutations in combination with herbicide use have been developed into in vitro plant selection systems (Lee et al. 2007; Sundar and Sakthivel 2008). Commercially available genetically modified crops with the selectable marker gene als are available for plant species such as carnation, cotton, canola, soybean, and maize (Miki and McHugh 2004). Previously, a mutant ALS allele from Arabidopsis (csr1-1) was used as a selectable marker in a rice transformation experiment (Li et al. 1992). Since the transgenic rice was both fertile and showed high resistance to chlorsulfuron, ALS inhibition and protein transport to the chloroplast are conserved between dicots and monocots, demonstrating that the gene can therefore be used as an effective selectable marker in monocots. Applicability for various crops was also confirmed by two studies which used a mutated form of the rice als gene as a selectable maker in rice and wheat genetic transformation (Ogawa et al. 2008; Endo et al. 2012). However, none of these als mutations have yet been tested and developed for herbicide selection in transgenic sugarcane production.
To date, sugarcane has only been genetically transformed for herbicide resistance with the pat (phosphinothricin acetyltransferase) gene, conferring resistance to phosphinothricin (Gallo-Meagher and Irvine 1996; Falco et al. 2000; Leibbrandt and Snyman 2003). Mutation breeding was also used to produce imidazolinone-resistant sugarcane which is effective against a wide spectrum of grass and broadleaf weeds (Koch et al. 2012). In this study, we therefore determined the sensitivity of sugarcane callus and plantlets to herbicides from the sulfonylurea and imidazolinone herbicide classes and report on the effectiveness of a mutated ALS as an in vitro selection system for the recovery of putative transgenic sugarcane callus.
Materials and Methods
Plant material and establishment of in vitro cultures.
In order to produce embryonic callus, the basal part of sugarcane (Saccharum spp. hybrids) cultivar NCo310 leaf roll, just above the apical meristem, was excised, cut into 2-mm-thick transverse sections, and placed on MS medium (Murashige and Skoog 1962; Highveld Biological, Johannesburg, South Africa) containing 2% (w/v) sucrose, 3 mg/l 2,4-dichlorophenoxyacetic acid (2,4-D), 0.5 g casein, and 0.22% (w/v) gelrite (Sigma-Aldrich, Johannesburg, South Africa), pH 6.0 (MS3). This was incubated for 6 wk at 24°C either in the dark or, if otherwise indicated, under a light regime of 16-h light/8-h dark.
Herbicide response curves.
The sensitivity of sugarcane callus toward chlorsulfuron, nicosulfuron, rimsulfuron, and the imadozolinone herbicides imazapyr, imazethapyr, and imazaquin was examined (Sigma-Aldrich). A known amount of sugarcane callus (0.25 g) was placed on basic MS3 medium (control) and compared to the callus placed on MS3 medium containing different concentrations of a single herbicide (between 3 and 500 μg/l). The sensitivity of the sugarcane callus to these herbicides was monitored for the following 15 wk with sub-culturing onto fresh medium every 2 wk. Callus embryogenicity and cell death were visually monitored and callus biomass determined by the increased weight of the starting material after 4 wk. The influence of various parameters, such as growth room light intensities, absence of light, and the presence/absence of casein in the growth medium, was also visually monitored and noted.
Sugarcane plantlets, regenerated from embryogenic callus, were kept at 25°C in natural light in the glasshouse and further sprayed with the six different herbicides at concentrations between 28 and 180 mg/l to determine ex vitro sugarcane sensitivity toward the different herbicides tested.
Isolation of tobacco mutant als gene.
Seeds of the rchl 6.6 chlorosulfuron-resistant tobacco line (Kochevenko and Willmitzer 2003) were germinated and allowed to grow for 4 wk. RNA was extracted from mature plant leaves using the method of Malnoy et al. (2001) and Hu et al. (2002). cDNA was synthesized from total RNA using the Fermentas RevertAid™ H First-strand cDNA synthesis kit according to the manufacturer’s specifications (Fermentas, Pretoria, South Africa).
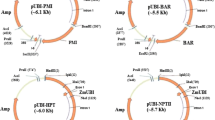
The primers designed for the tobacco als coding sequence (accession no. X07645) were ALS FW (5′-AAGGATCCTTCGTCTCTCACT-3′) and ALS REV (5′-AACCTAGGAGCTCTGTAGCAC-3′) for PCR amplification from the cDNA template. These primers included the BamHI restriction sites on both ends of the PCR product. PCR was performed using the pfu DNA polymerase enzyme (Fermentas) according to the manufacturer’s specifications. The 2-kb als PCR product was purified, sequenced, and ligated into the BamHI site of the pUBI510 plant expression vector under control of the maize ubiquitin promoter and nos terminator (pUBI-als).
Biolistic gene transfer, selection, and regeneration of plants.
Embryogenic callus explants were placed on an osmoticum medium consisting of the basic MS3 medium and 0.2 M of each sorbitol and mannitol (Sigma Aldrich) 4 h prior to and after bombardment (Vain et al. 1993).
Sugarcane callus was transformed using a biolistic particle delivery system as previously described by Franks and Birch (1991), with some modifications. Sterilized tungsten particles (M10; Bio-Rad, Hercules, CA) were mixed with 10 μl plasmid DNA (1 μg/μl), 50 μl 2.5 M CaCl2, and 20 μl 0.1 M spermidine (Sigma Aldrich). Particle inflow gun was constructed locally and was used for all biolistic DNA delivery. For bombardment, 5 μl of the particle suspension was placed into the center of a 1-mm2 metal grid above the explants. Target tissue was placed 16.5 cm below the particle source and the chamber was evacuated to a pressure of 80 kPa before the particles were discharged.
Bombarded tissue was maintained on MS3 medium for 3 d before selection started on predetermined concentrations of chlorsulfuron for 8–10 wk in the dark with sub-culturing onto fresh medium every 2 wk. Tissue was regenerated on herbicide-containing MS medium, lacking 2,4-D, in the light for 8 wk or until roots formed. Calli were regenerated into plantlets on rooting media containing chlorsulfuron to further eliminate non-transgenic clones. Plantlets, 5 cm high with roots, were ex vitro-acclimatized in soil-containing pots in the glasshouse. Surviving 2-wk-old plantlets were sprayed with predetermined concentrations of herbicide (100 mg/l chlorsulfuron) to reduce the number of non-transgenic plants that escaped the in vitro selection regime.
PCR analysis of putative transgenic plants.
Putative transformed plants were analyzed for the presence of the als transgene using PCR where one primer binds in the ubiquitin promoter region (Ubi-exp: 5′-ATACGCTATTT ATTTGCTTGG-′3) and the second primer in the als gene (ALS REV). Approximately 200 ng of the genomic DNA template was used for a 50-μl PCR reaction. Genomic DNA was isolated according to the manufacturer’s instruction of the Fermentas GeneJet Plant Genomic DNA purification kit (Fermentas).
For gene expression analysis, 500 ng of total RNA was extracted from putative transgenic sugarcane leaf material by the method of Malnoy et al. (2001) and Hu et al. (2002). cDNA was synthesized from total RNA with the Fermentas RevertAidTM H First-strand synthesis cDNA kit according to the manufacturer’s specifications (Fermentas). Tobacco als cDNA-specific primers (forward: 5′-CAATGGGAGGATCGG TTCTA-′3; reverse: 5′-CAAGTATGGCCCAGGAGTGT-′3) and sugarcane actin cDNA primers, as internal controls (forward: 5′-TCACACTTTCTACAATGAGCT-3′; reverse” 5′-GATATCCACATC ACACTT CAT-3′), were used for amplification.
Results
Herbicide sensitivity.
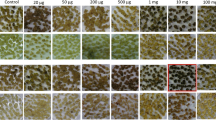
Sugarcane callus was placed on different concentrations of sulfonylurea and imidazolinone herbicides. Those tested included the sulfonylurea herbicides—chlorsulfuron, nicosulfuron, and rimsulfuron—and the imadozolinone herbicides—imazapyr, imazethapyr, and imazaquin. Herbicide concentrations varied between 3 and 500 μg/l. The absence/presence of light had little influence on herbicide activity (Figs. 1 and 2). Sugarcane callus placed in direct light showed severe signs of stress due to the production of phenolic compounds in the cells (visible as a purple coloration in the cells). However, this was not related to the presence of herbicide in the growth medium (Fig. 1). Furthermore, the presence or absence of casein in the growth medium did not significantly influence the sensitivity of the sugarcane callus toward the herbicides (data not shown). Although imadozolinone-type herbicides had very little effect on the growth of sugarcane callus (data not shown), inhibition was found on medium containing chlorsulfuron and rimsulfuron at 3.6 and 200 μg/l, respectively (Table 1 and Figs. 1 and 2). Callus placed on the control MS3 medium showed on average a 10.5-fold increase in fresh weight over 4 wk (0.25–2.63 g), while that placed on chlorsulfuron or rimsulfuron showed no, or at most, a 5-fold increase in weight over 4 wk, respectively (Fig. 2). Prolonged exposure of the callus (more than 12 wk) to the different herbicides did not increase the sensitivity response already obtained during the first 8 wk; rather, it tended to reduce the effect of the initial exposure. Based on visual observations and weight measurements, inhibition of callus growth was most obvious between 6 and 8 wk after callus initiation on chlorsulfuron- or rimsulfuron-containing media (Figs. 1 and 2). Although the exposure of callus to any of the six herbicides did not result in cell death, it did cause loss of callus embryogenesis and a reduction in biomass.
Sugarcane callus growth on basic MS3 medium (control) compared to the medium supplemented with different concentrations of chlorsulfuron, placed under filtered light. Observations shown were recorded after 5 wk on chlorsulfuron. Square indicates treatment (3.6 μg/l chlorsulfuron) resulting in the most severe callus growth inhibition.
Sugarcane callus growth on basic MS3 medium (control) compared to the medium supplemented with different concentrations of chlorsulfuron (A–D) and rimsulfuron (E), placed in the dark. Observations were done after 5 wk (A), 10 wk (B, E), and 15 wk (C) on herbicide. D. A close-up view of the callus growth after 10 wk on 0 (control), 3.6, and 20 μg/l chlorsulfuron-containing medium. Arrows indicated treatments resulting in the most severe callus growth inhibition on chlorsulfuron (3.6 μg/l) or rimsulfuron (200 μg/l).
Sugarcane plantlets were further exposed to different herbicide concentrations between 28 and 180 mg/l, and these showed very low sensitivity to the imidazolinone class of herbicides. Sugarcane plantlets started dying on 100 mg/l of chlorsulfuron and rimsulfuron after 4 wk (Fig. 3). Plant death could, however, be accelerated to 3 wk post-exposure when the herbicide concentration was increased to 140 mg/l.
Analysis of putative transgenic plants which survived the in vitro selection regime. A, PCR amplification of a fragment of the fused ubiquitin promoter (ubi) and mutant acetolactate synthase B (alsb) gene from gDNA extracted from putative transformed pUBI-als sugarcane plants. Lane M, λDNA digested with PstI; H 2 0, water control; C control Nco310 sugarcane cultivar, Pos positive control, pUBI-als vector. Lines 1, 3, 5–8 were positive transgenic plants. Lines 2 and 4 were negative. B, Four weeks after putative transgenic plants were sprayed with 100 mg/l of chlorsulfuron in the glasshouse. Lines 1, 3, 5–8 survived the herbicide spraying. Lines 2 and 4 died after chlorsulfuron spraying (white arrows). Lines correspond to the lanes indicated in the PCR analysis.
ALS isolation and transfer into sugarcane callus.
The mutant ALS SurB isoform (alsb) was isolated from tobacco cDNA (Kochevenko and Willmitzer 2003). The PCR product was sequenced and a single nucleotide change at base pair 1,719 (TGG to TTG), which results in a Trp-573-Leu amino acid substitution, was confirmed. The insert was ligated in a “sense” orientation with respect to the promoter into the BamHI restriction site of the pUBI 510 plant expression vector, resulting in the vector pUBI-als.
The plant expression vector pUBI-als was bombarded into embryogenic sugarcane callus using 12 plates. Each plate contained a 2.5-cm diameter circle of callus closely stacked together. Bombarded calli were placed on medium containing 3.6 μg/l chlorsulfuron as a selection agent (amount as determined by a herbicide kill curve). Bombarded callus was placed in the dark for 8 wk with sub-culturing onto fresh medium after 2, 4, and 6 wk. Following selection, putative transformed calli were transferred to the light on a regeneration medium lacking 2,4-D. After 8 wk on chlorsulfuron selection, up to 20% of the bombarded calli survived the treatment and showed active growth with a healthy yellow color compared to non-surviving dormant and grayish calli. The herbicide had little effect on the rooting ability of the putative transgenic clones. Regenerated plantlets were transferred to pots containing soil in the greenhouse for ex vitro acclimatization. After 2 wk, 30 putative transgenic plantlets were established in the glasshouse. These plants were sprayed with 100 mg/l chlorsulfuron, which reduced the number of plantlets by ∼70%, to eight surviving plants. The surviving putative transgenic plants were tested for the presence of the alsb gene via PCR. Six lines were found to be positively transgenic for the maize ubiquitin promoter (Ubi-1)–alsb fusion gene (Fig. 3). In five of these transgenic lines, the tobacco als gene transcript was verified by reverse transcription polymerase chain reaction (Fig. 4).
PCR amplification using sugarcane cDNA as the template to determine gene expression. (A) Internal standard as amplified with sugarcane actin primers. (B) Expression of the tobacco als gene in sugarcane leaves. Lane M, λDNA digested with PstI. H 2 0, water control; C, control Nco310 sugarcane cultivar. Lanes 1–5 represent five transgenic sugarcane clones containing the tobacco als gene insert.
Discussion
In this study, a mutated als gene was successfully used for the first time as a selectable marker in combination with the herbicide chlorsulfuron as a selection agent, resulting in the production of transgenic sugarcane lines resistant against the herbicide. The first step in our study toward developing the ALS selection system in sugarcane was to determine the sensitivity of the sugarcane callus toward a range of ALS targeting herbicides. Callus biomass proliferation was significantly inhibited, up to 10-fold, when exposed to the different herbicides, in particular chlorsulfuron. However, the growth response did not always correlate with an increased herbicide concentration. For example, chlorsulfuron was the most effective herbicide to inhibit callus growth at a concentration of 3.6 μg/l (10 nM), while higher concentrations, such as 50 μg/l chlorsulfuron, did not affect growth at all. A similar low concentration of chlorsulfuron (10 nM) has previously been found to effectively inhibit transgenic rice protoplast growth (Li et al. 1992). On the other hand, chlorsulfuron-resistant maize calli were selected on 50 nM chlorsulfuron, while soybean embryos were only sensitive to ALS herbicides (mix of primisulfuron and prosulfuron) when a concentration as high as 183 mg/l was applied (Fromm et al. 1990; Rao et al. 2009).
In our study, not all of the herbicides affected sugarcane callus growth. Imadozolinone-type herbicides had very little or no effect on the growth of Nco310 sugarcane callus. This is in contrast to a recent study done with N19 sugarcane callus where regeneration was suppressed when callus was exposed to imazapyr concentrations as low as 0.08 μM (21 μg/l; Koch et al. 2012). The concentrations of imazapyr, imazethapyr, or imazaquin as high as 500 μg/l scarcely affected sugarcane callus biomass and embryogenicity and also did not result in cell death. This is similar to the result found by Heering et al. (1992), where wheat calli were less sensitive to 100 μM imazapyr, in contrast to the whole wheat plant. Therefore, the effectiveness of herbicide concentrations on growth differs significantly across plant tissues and plant species.
The ALS selection system was less effective in sugarcane when compared to other well-established systems, such as the nptII gene and use of geneticin. In the past, when the geneticin/nptII system was applied for selecting transgenic sugarcane calli, 1–5% of the total bombarded calli would survive with almost no escapes (Bower and Birch 1992; Snyman et al. 1996). In our chlorsulfuron/als system, however, up to 20% of the bombarded calli survived after 8 wk of in vitro selection with insufficient new growth to easily differentiate between transgenic and non-transgenic calli. Also, from the surviving calli, 30 putative transgenic plants regenerated, of which six contained the als gene, an escape rate of 20%. Escapes could be dramatically reduced in our study by ∼70% with ex vitro herbicide spraying. Similar to the escape rate for sugarcane, the ALS selectable marker system applied in wheat had an escape rate of 7.7–60% of surviving embryos not containing the als selectable marker gene (Ogawa et al. 2008). Therefore, based on the escape rate of the chlorsulfuron/als system reported here, it seems to be comparable to ALS systems developed for other crops, but less efficient than the geneticin/nptII system in sugarcane.
A disadvantage of sulfonylurea and imidazolinone herbicide application for selection might be the lack of induced cell death. In this study, exposure of callus to these herbicides only resulted in biomass reduction and loss of embryogenesis. A similar result has been reported for the mannose selection system in sugarcane where different concentrations of mannose inhibited growth by 50–80%, but could not completely suppress callus growth (Jain et al. 2007). Also, wheat callus exposed to imazapyr only showed 50% growth inhibition after 70 d. (Heering et al. 1992).
Our results have further shown that light was not required to ensure herbicide activity. Since callus cultured under light turned purple due to excessive accumulation of anthocyanin pigments as a result of light stress, application of the selection system should preferably be done in the dark. In the dark, coloration changes included a change from a healthy pale yellow to a grayish white, and shades in between. Very long exposure to the different herbicides beyond 6–8 wk also did not result in greater growth inhibition of sugarcane callus or cell death as callus seems to overcome the inhibitory growth effect of the herbicides.
Overall, in this study, the expression of the als gene, together with chlorsulfuron as a selection agent, was successfully used to produce transgenic sugarcane plants. The in vitro selection system was complimented and further improved by a single ex vitro herbicide spray application of surviving plantlets. Since a large variation exists in the sensitivity of plant tissues to different herbicides, in the future, it may be useful to also test additional herbicide classes, such as pyrimidinylcarboxylates, which also target the als gene, in sugarcane. In general, applying ALS in combination with sulfonylurea or imidazolinone herbicides for the selection of transgenic plants has several advantages. Firstly, these herbicides have little or no known toxic side effects on animals and no measurable mutagenic properties (Levitt 1983; Powles and Yu 2010). In addition, the use of a mutated als gene is less likely to prompt concerns regarding food safety in GM plants because a native als gene exists in all plants. The mutated als gene can also act both as an in vitro selectable marker gene during sugarcane genetic transformation as well as a genetic insertion event that conveys resistance to herbicides in the field during crop production. In this regard, a sugarcane crop with a herbicide resistance trait will supply much needed protection against weeds during crop production. It has been shown that weeds can reduce sugarcane yields by more than 40%, greatly increasing the cost of production (Millhollon 1992, 1995). ALS-inhibiting herbicides are also used for weed control in the South African sugar industry, and these ALS-inhibiting herbicides account for about 17.5% of the total global herbicide market (Green 2007). Introduction of a herbicide-resistant sugarcane would not only provide a useful selection system but would ultimately also be beneficial to sugarcane producers.
References
Bae C, Young-Ill L, Yong-Pyo L, Yong-Won S, Do-Jin L, Deuk-Chum Y, Hyo-Yeon L (2002) Selection of herbicide tolerant cell lines from γ-ray-irradiated cell cultures in rice (Oryza sativa L. cv. Ilpumbyeo). J Plant Biotechnol 4:123–127
Bower R, Birch R (1992) Transgenic sugarcane plants via micro-projectile bombardment. Plant J 2:409–416
Chaleff RS, Mauvais CJ (1984) Acetolactate synthase is the site of action of two sulfonylurea herbicides in higher plants. Science 224:1443–1445
Chaleff RS, Ray TB (1984) Herbicide resistant mutant from tobacco cell cultures. Science 223:1148–1161
Chengalrayan K, Gallo-Meagher M, English RG (2001) Novel selection agents for sugarcane transformation. Soil Crop Sci Soc Florida Proc 60:81–87
Chern WS, Rickertsen K, Tsuboi N, Fu TT (2002) Consumer acceptance and willingness to pay for genetically modified vegetable oil and salmon: a multiple-country assessment. AgBioForum: J Agrobiotechnol Manag Economics 5:105–112. http://www.agbioforum.org. Accessed 1 November 2012
Corbett CL, Tardif FJ (2006) Detection of resistance to acetolactate synthase inhibitors in weeds with emphasis on DNA-based techniques: a review. Pest Manag Sci 62:584–597
Duggleby RG, Pang SS (2000) Acetohydroxyacid synthase. J Biochem Mol Biol 33:1–36
Endo M, Shimizu T, Toki S (2012) Selection of transgenic rice plants using a herbicide tolerant form of the acetolactate synthase gene. Methods Mol Biol 847:59–66
Enriquez-Obregon GA, Nazquez PR, Prieto SD, Riva-Gustavo AD, Selmann HG (1998) Herbicide resistant sugarcane (Saccharum officinarum L.) plants by Agrobacterium-mediated transformation. Planta 206:20–27
Falco MC, Neto AT, Ulian EC (2000) Transformation and expression of a gene for herbicide resistance in a Brazilian sugarcane. Plant Cell Rep 19:1188–1194
Falco SC, Dumas KS (1985) Genetic analysis of mutants of Saccharomyces cerevisae resistant to the herbicide sulfometuron methyl. Genetics 1099:21–35
Franks JR (1999) The status and prospects for genetically modified crops in Europe. Food Policy 24:565–584
Franks T, Birch RG (1991) Gene transfer into intact sugarcane callus using microprojectile bombardment. Aus J Plant Physiol 18:471–480
Fromm ME, Morrish F, Armsoron C, Williams R, Thomas J, Klein TM (1990) Inheritance and expression of chimeric genes in the progeny of transgenic maize plants. Biotechnology 8:833–839
Gallo-Meagher M, Irvine JM (1996) Herbicide resistant transgenic sugarcane plants containing the bar gene. Crop Sci 36:1367–1374
Green JM (2007) Review of glyphosate and ALS-inhibiting herbicide crop resistance and resistant weed management. Weed Technol 21:547–558
Heering DC, Guenzi AC, Peeper TF, Claypool PL (1992) Growth response of wheat (Triticum aestivum) callus to imazapyr and in vitro selection of resistance. Weed Sci 40:174–179
Hu CG, Honda C, Kita M, Zhang Z, Tsuda T, Moriguchi T (2002) A simple protocol for RNA isolation from fruit trees containing high levels of polysaccharides and polyphenol compounds. Plant Mol Biol Rep 20:69/1–69/7
Jain M, Chengalrayan K, Abouzid A, Gallo M (2007) Prospecting the utility of a PMI/mannose selection system for the recovery of transgenic sugarcane (Saccharum spp. hybrid) plants. Plant Cell Rep 26:581–590
Joersbo M, Okkels FT (1996) A novel principle for selection of transgenic plant cells: positive selection. Plant Cell Rep 16:219–221
Joyce P, Kuwahata M, Turner N, Lakshmanan P (2010) Selection system and co-cultivation medium are important determinants of Agrobacterium-mediated transformation of sugarcane. Plant Cell Rep 29:173–183
Khruangchan N, Chatchawankanphanich O, Pornprom T (2011) Acetolactate synthase gene mutation conferring imazapyr tolerance in sugarcane clone. NRCT J 41:115–127
Koch AC, Ramgareeb S, Rutherford RS, Snyman SJ, Watt MP (2012) An in vitro mutagenesis protocol for the production of sugarcane tolerant to the herbicide imazapyr. In Vitro Cell Dev Biol—Plant 48:417–427. doi:10.1007/s11627-012-9448-x
Kochevenko A, Willmitzer L (2003) Chimeric RNA/DNA oligonucleotide-based site-specific modification of the tobacco acetolactate synthase gene. Plant Physiol 132:174–184
LaRossa RA, Schloss JV (1984) The sulfonylurea herbicide sulfometuron methyl is an extremely potent and selective inhibitor of acetolactate synthase in Salmonell typhimurium. J Biol Chem 259:8753–8757
Laplante J, Rajcan I, Tardif FJ (2009) Multiple allelic forms of acetohydroxyacid synthase are responsible for herbicide resistance in Setaria viridis. Theor Appl Genet 119:577–586
Lee K, Yang K, Kang K, Kang S, Lee N, Back K (2007) Use of Myxococcus xanthus protoporphyrinogen oxidase as a selectable marker for transformation of rice. Pest Biochem Physiol 88:31–35
Lee KY, Townsend J, Tepperman J, Black M, Chui CF, Mazur B, Dunsmuir P, Bedbrook J (1988) The molecular basis of sulfonylurea herbicide resistance in tobacco. EMBO J 7:1241–1248
Leibbrandt NB, Snyman SJ (2003) Stability of gene expression and agronomic performance of a transgenic herbicide-resistant sugarcane line in South Africa. Crop Sci 43:671–678
Levitt G (1983) Sulfonylureas, new herbicides. In: Miyamoto J, Keraney PC (eds) Pesticide chemistry, human welfare and the environment. Oxford University Press, New York, pp 243–250
Li Z, Hayashimoto A, Murai N (1992) A sulonylurea herbicide resistance gene from Arabidopsis thaliana as a new selectable marker for production of fertile transgenic rice plants. Plant Physiol 100:662–668
Malnoy M, Reynoird JP, Mourgues F, Chevreau E, Simoneau P (2001) A method for isolating total RNA from pear leaves. Plant Mol Biol Rep 16:69
Mazur BJ, Falco SC (1989) The development of herbicide resistant crops. Annu Rev Plant Physiol Plat Mol Biol 40:441–470
McCourt JA, Pang SS, King-Scott J, Guddat LW, Dugglebly RG (2006) Herbicide-binding sites revealed in the structure of plant acetohydroxyacid synthase. Proc Natl Acad Sci U S A 103:569–573
Miki B, McHugh S (2004) Selectable marker genes in transgenic plants: applications, alternatives and biosafety. J Biotechnol 107:193–232
Millhollon RW (1992) Effect of itchgrass (Rottboellia cochinchinensis) interference on growth and yield of sugarcane (Saccharum spp. hybrids). Weed Sci 40:48–53
Millhollon RW (1995) Growth and yield of sugarcane as affected by johnsongrass (Sorgum halepense) interference. J Am Soc Sugarcane Technol 15:32–40
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Newhouse KE, Singh BK, Shaner DL, Stidham M (1991) Mutations in corn (Zea mays) conferring resistance to imidazolinones. Theor Appl Genet 83:65–70
Newhouse KE, Smith W, Starret M, Schaefer T, Singh BK (1992) Tolerance to imidazolinone herbicides in wheat. Plant Physiol 100:882–886
Ogawa T, Kawahigashi H, Toki S, Handa H (2008) Efficient transformation of wheat by using a mutated rice acetolactate synthase gene as a selectable marker. Plant Cell Rep 27:1325–1331
Powles SB, Yu Q (2010) Evolution in action: plants resistant to herbicides. Annu Rev Plant Biol 61:317–347
Punyadee P, Thongros M, Pornprom T (2007) Biochemical mechanism of resistance to imazapyr in sugarcane cell selections. Thai J Agric Sci 40:133–141
Rajasekaran K, Grula JW, Anderson DM (1996) Selection and characterization of mutant cotton (Gossypium hirustum L.) cell lines resistant to sulfonylurea and imidazolinone herbicides. Plant Sci 199:115–124
Rao SS, Mamodou L, McConnell M, Polisetty R, Kwanyuen P, Hildebrand D (2009) Non-antibiotic selection systems for soybean somatic embryos: the lysine analog aminoethyl-cysteine as a selection agent. BMC Biotechnol 9:94
Sebastian SA, Fader MG, Ulrich FJ, Forney RD, Chaleff SR (1989) Semidominant soybean mutation for resistance to sulfonylurea herbicides. Crop Sci 199:115–124
Schloss JV, Van Dyk DE, Vasta JF, Kutny RM (1985) Purification and properties of Salmonella typhimurium acetolactate synthase isozyme II from Escherichia coli HB101/pDU9. Biochemistry 24:4952–4959
Schnell J, Labbe H, Kovinich N, Manabe Y, Miki B (2012) Comparability of imazapyer-resistant Arabidopsis created by transgenesis and mutagenesis. Transgenic Res 21:1255–1264. doi:10.1007/s112480129597z
Singh BK, Shaner DL (1995) Biosynthesis of branched amino acids: from test tube to field. Plant Cell 7:935–944
Snyman SJ, Meyer GM, Carson DL, Botha FC (1996) Establishment of embryogenic callus and transient gene expression in selected sugarcane varieties. S Afr J Bot 62:151–154
Sundar IK, Sakthivel N (2008) Advances in selectable marker genes for plant transformation. J Plant Physiol 165:1698–1716
Swanson EB, Hergesell MJ, Arnoldo M, Sippel DW, Wong RSC (1989) Microspore mutagenesis and selection: canola plants with field tolerance to imidazolinones. Theor Appl Genet 78:525–530
Environmental Protection Agency US (1994) Neomycin phosphotransferase: II. Tolerance exemption. Fed Regist 56:4935
Vain P, McMullen MD, Finer JJ (1993) Osmotic treatment enhances particle bombardment-mediated transient and stable transformation of maize. Plant Cell Rep 12:84–88
Warwick SI, Xu R, Sauder C, Beckie HJ (2008) Acetolactate synthase target-site mutations and single nucleotide polymorphism genotyping in ALS-resistant kochia (Kochia scoparia). Weed Sci 56:797–806
Wright T, Penner D (1998) Cell selection and inheritance of imidazolinone resistance in sugar beet (Beta vulgaris). Theor Appl Genet 96:612–620
Yadav N, McDevitt RE, Benard S, Falco SC (1986) Single amino acid substitutions in the enxyme acetolactate synthase confer resistance to the herbicide sulfometuron methyl. Proc Natl Acad Sci U S A 83:4418–4422
Acknowledgments
We would like to thank Prof. Lothar Willmitzer (Max Planck Institute of Molecular Plant Physiology, Germany) for providing us with seeds from the rchl 6.6 chlorosulfuron-resistant tobacco line. This project was funded by Biosafety, South Africa (www.biosafety.org.za).
Author information
Authors and Affiliations
Corresponding author
Additional information
Editor: J. Foster
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
van der Vyver, C., Conradie, T., Kossmann, J. et al. In vitro selection of transgenic sugarcane callus utilizing a plant gene encoding a mutant form of acetolactate synthase. In Vitro Cell.Dev.Biol.-Plant 49, 198–206 (2013). https://doi.org/10.1007/s11627-013-9493-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11627-013-9493-0