Abstract
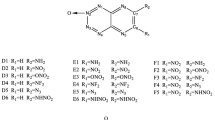
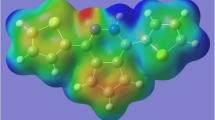
The polyazoles have five-membered heterocyclic rings with N/C ratios of at least 2/3. Their high nitrogen contents result in relatively high crystal densities and large positive heats of formation, features that make them attractive as frameworks for energetic materials. However the presence of linked nitrogens (catenation) is accompanied by reduced stability (reflected in the large heats of formation). Several related factors may be involved in this, including the weakness of N-N bonds, the possibility of decomposing through release of the very stable N2(g) and the repulsion between lone pairs of neighboring nitrogens. We show that in the polyazoles a particularly important source of reduced stability is the presence of adjacent doubly-coordinated nitrogens, especially if connected by a formal double bond. Our computed polyazole electrostatic potentials are consistent with significant repulsion between the lone pairs of these nitrogens. Introducing an N-oxide linkage on one of them leads to some stabilization; the oxygen withdraws electronic charge from the heterocyclic ring, reducing the electronic repulsion within it. However the N-oxide derivatives having more nitrogen catenation are still the less stable ones. Comparing the polyazoles to the polyazines (six-membered N/C heterocyclic rings), the π electrons in the former are less delocalized and the computed bond lengths in the polyazole rings are consistent with the formal single and double bonds in their Lewis structures. An interesting consequence is that if a second N-oxide is introduced next to the original one, the bond between the two nitrogens remains intact in polyazoles if it is formally double but it is considerably weakened or broken in polyazines, and in polyazoles if formally single.





Similar content being viewed by others
References
Afeefy HY, Liebman JF, Stein SF (1998) Neutral thermochemical data. In: Linstrom PJ, Mallard WG (eds) NIST Chemistry Webbook, NIST Standard Reference Database No.69, Gaithersburg. http://webbook.nist.gov
Awadallah AM, Zahra JA (2008) Molecules 13:170–176
Balabin RM (2009) J Chem Phys 131:154307(1–11) and references cited
Mohite PB, Bhaskar VH (2011) Internat J PharmaTech Res 3:1557–1566
Kumar R, Yar MS, Chaturvedi S, Srivastava A (2013) Internat J PharmaTech Res 5:1844–1869
Sobol RW (2012) Encyclopedia of cancer. Springer, Berlin, p. 36
Kumar R, Singh AD, Singh J, Singh H, Roy RK, Chandhary A (2014) Mini-Rev Med Chem 14:72–83
Benson FR (1984) The high nitrogen compounds. Wiley-Interscience, New York
Katritzky AR (ed) (1985) Handbook of heterocyclic chemistry. Pergamon Press, Oxford
Boyer JH (1986) Nitroazoles. The C-nitro derivatives of five-membered N- and N,O- heterocycles, VCH, Deerfield Beach, FL
Klapötke TM (2007) Struct Bond 125:85–121
Gao H, Shreeve JM (2011) Chem Rev 111:7377–7436
Aroní G, Barrios LA, Roubeau O, Gamez P (2011) Coord Chem Rev 255:485–546
Klapötke TM (2011) Chemistry of high-energy materials. de Gruyter, Berlin
Licht HH, Ritter H (1994) J Energ Mater 12:223–235
Pagoria PF, Lee GS, Mitchell AR, Schmidt RD (2002) Thermochim Acta 384:187–204
Yu Z, Bernstein ER (2013) J Phys Chem A 117:10889–10902
Yin P, Zhang Q, Shreeve JM (2016) Acc Chem Res 49:4–16
Kamlet MJ, Jacobs SJ (1968) J Chem Phys 48:23–35
Rice BM, Hare J (2002) Thermochim Acta 384:377–391
Politzer P, Murray JS (2011) Central Eur J Energ Mater 8:209–220
Politzer P, Murray JS (2015) J Mol Model 21:262(1–11)
Politzer P, Murray JS (2016) Propell Explos Pyrotech 41:1–13
Stine JR (1990) J Energ Mater 8:41–73
Gavezzotti A (1989) J Am Chem Soc 111:1835–1843
Rice BM, Byrd EFC (2013) J Comput Chem 34:2146–2151
Luo Y-R (2003) Handbook of bond dissociation energies in organic compounds. CRC Press, Boca Raton, FL
Rheingold AL (ed) (1977) Homoatomic rings, chains and macromolecules of main-group elements. Elsevier, Amsterdam
Cotton FA, Wilkinson G, Murillo CA, Bochmann M (1999) Advanced inorganic chemistry, 6th edn. Wiley-Interscience, Chichester
Wright JS (1974) J Am Chem Soc 96:4753–4760
Fabian J, Lewars E (2004) Can J Chem 82:50–69
Noyman M, Zilberg S, Haas Y (2009) J Phys Chem A 113:7376–7382
Politzer P, Lane P, Murray JS (2013) Struct Chem 24:1965–1974
Zhao X, Qi C, Zhang R, Zhang S, Li S, Pang S (2015) J Mol Model 21(1–6):223
Begtrup M, Nielsen CJ, Nygaard L, Samdal CE, Sjøgren CE, Sørensen GO (1988) Acta Chem Scand 42A:500–514
Novak L, Kovač B, Klasinc L, Ostrovskii VA (2003) Spectrochim Acta, Part A 59:1725–1731
Wang Y, Wu JI-C, Li Q, Schleyer PR (2010) Org Lett 12:4824–4827
March J (1985) Advanced organic chemistry, 3rd edn. Wiley-Interscience, New York
Taft RW, Anvia F, Taagepera M, Catalán J, Elguero J (1986) J Am Chem Soc 108:3237–3239
Tomas F, Abboud J-LM, Laynez J, Notario R, Santos L, Nilsson SO, Catalán J, Claramunt RM, Elguero J (1989) J Am Chem Soc 111:7348–7353
Wilson KJ, Perera SA, Bartlett RJ, Watts JD (2001) J Phys Chem A 105:7693–7699
Cremer D, Wu A, Larsson A, Kraka E (2000) J Mol Model 6:396–412
Cremer D, Kraka E (2010) Curr Org Chem 14:1524–1560
Macaveiu L, Göbel M, Klapötke TM, Murray JS, Politzer P (2010) Struct Chem 21:139–146
Storm CB, Ryan RR, Ritchie JP, Hall JH, Bachrach SM (1989) J Phys Chem 93:1000–1007
Politzer P, Grice ME, Seminario JM (1997) Internat J Quantum Chem 61:389–392
Murray JS, Politzer P (1988) Chem Phys Lett 152:364–370
Murray JS, Politzer P (1987) Chem Phys Lett 136:283–288
Ramsden CA (2010) Tetrahedron 66:2695–2699
Churakov AM, Tartakovsky VA (2004) Chem Rev 104:2601–2616
Huynh M-HV, Hiskey MA, Hartline EL, Montoya DP, Gilardi R (2004) Angew Chem Int Ed 43:4924–4928
Chavez DE, Hiskey MA, Huynh MH, Naud DL, Son SF, Tappan BC (2006) J Pyrotech 23:70–80
Lai W, Lian P, Ge Z, Liu Y, Yu T, Lv J (2016) J Mol Model 22:83(1–11)
Ali AN, Son SF, Hiskey MA, Naud DL (2004) J Propuls Power 20:120–126
Huynh MHV, Hiskey MA, Chavez DE, Gilardi RD (2005) J Energ Mater 23:99–106
Fischer D, Klapötke TM, Piercey DG, Stierstorfer J (2013) Chem Eur J 19:4602–4613
Politzer P, Lane P, Murray JS (2014) Mol Phys 112:719–725
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, et al. (2009) Gaussian 09, Revision A.1. Gaussian, Inc., Wallingford, CT
Bader RFW, Carroll MT, Cheeseman JR, Chang C (1987) J Am Chem Soc 109:7968–7979
Stewart RF (1979) Chem Phys Lett 65:335–342
Politzer P, Truhlar DG (eds) (1981) Chemical applications of atomic and molecular electrostatic potentials. Plenum Press, New York
Klein CL, Stevens ED (1988) In: Liebman JF, Greenberg A (eds) Structure and reactivity, vol ch 2. VCH Publishers, New York, pp. 25–64
Murray JS, Politzer P (2011) WIREs Comput Mol Sci 1:153–163
Bulat FA, Toro-Labbé A, Brinck T, Murray JS, Politzer P (2010) J Mol Model 16:1679–1693
Allen FH, Kennard O, Watson DG, Brammer L, Orpen AG, Taylor R (1987) J Chem Soc Perkin Trans II:S1–S19
Cyranski MK, Krygowski TM, Katritzky AR, Schleyer PR (2002) J Org Chem 67:1333–1338
Krygowski TM, Oziminski WP, Ramsden CA (2011) J Mol Model 17:1427–1433
Dabbagh HA, Rasti E, Chermahini AN (2010) J Mol Struct (THEOCHEM) 947:92–100
Katritzky AR, Taylor R (1990) Adv Heterocyclic Chem 47:277–323
Lane P, Murray JS, Politzer P (1991) J Mol Struct (THEOCHEM) 236:283–296
Poole JS (2009) J Mol Struct (THEOCHEM) 894:93–102
Tang Z-X, Li X-H, Zhang X-Z (2009) J Mol Struct (THEOCHEM) 907:126–130
Rowland FS, Taylor R (1996) J Phys Chem 100:7384–7391
Alvarez S (2013) Dalton Trans 42:8617–8636
Murray JS, Seminario JM, Politzer P (1989) J Mol Struct (THEOCHEM) 187:95–108
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Politzer, P., Murray, J.S. Computational analysis of polyazoles and their N-oxides. Struct Chem 28, 1045–1063 (2017). https://doi.org/10.1007/s11224-016-0909-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-016-0909-4