Abstract
Aims
Because plants cannot change their environmental circumstances by changing their location, they must instead adapt to a wide variety of environmental conditions, especially soil conditions. One of the most effective ways for a plant to adapt to a given soil condition is by modifying its root system architecture. We aim to identify the genetic factors controlling root growth angle, a trait that affects root system architecture.
Methods
The present study consisted of a genetic analysis of the seminal root growth angle in wheat; the parental varieties of the doubled haploid lines (DHLs) used in this study exhibited significantly different root growth directions. Using the ‘basket’ method, the ratio of deep roots (DRR; the proportion of total roots with GA > 45 degrees) was observed for evaluating deep rooting.
Results
We were able to identify novel quantitative trait loci (QTLs) controlling the gravitropic and hydrotropic responses of wheat roots. Moreover, we detected one QTL for seminal root number per seedling (RN) on chromosome 5A and two QTLs for seminal root elongation rate (ER) on chromosomes 5D and 7D.
Conclusions
Gravitropic and hydrotropic responses of wheat roots, which play a significant role in establishing root system architecture, are controlled by independent genetic factors.
Similar content being viewed by others
Introduction
Roots, the hidden part of a plant, play several essential roles in the plant life cycle. They are important not only for the uptake of nutrients and water but also for environmental stress tolerance. Therefore, plants have several ways to modify their root system architecture in response to changes in the external environment (Osmont et al. 2007). Analyses of genetic factors contributing to root system architecture are still very limited, however, partly because of the difficulty of observing the distribution of roots in field conditions, and partly because of the complexity of the effects of environmental conditions on root system architecture.
To overcome these difficulties, researchers have been working on the relationship between root characteristics at the seedling stage and root system architecture in the field. Oyanagi (1994) measured the root angle (Θ) in 7-day-old seedlings of several wheat cultivars using the basket method. Θ is the angle between the horizontal and a line connecting the root tip to the base root. He also grew the same cultivars in an upland field with soil type light-colored andosol, in Tsukuba, Japan, and then they measured the root depth index at the stem elongation stage. He found that Θ is closely correlated (r = 0.779, P < 0.05) with the root depth index. Based on this experiment, they suggested that cultivars which show large root angles as seedlings have deep root system architecture at the stem elongation stage. He also found that Θ was larger in northern Japanese cultivars, which had a deep root system than the southern Japanese cultivars which have a shallow root system. From these results, he suggested that the seminal root growth angle is useful for predicting vertical root distribution. By comparing the results obtained using a gel-filled chamber to measure root growth angle at the seedling stage and a soil-filled chamber at the 4–5 leaf stage (40 days after planting), Manschadi et al. (2008) have also suggested that selection for seminal root traits might be useful in breeding to increase the drought tolerance of wheat varieties with deep root architecture. They conducted that selection for root growth angle and number of seminal roots may help to identify genotypes with root system architecture adapted to drought tolerance. In rice, Kato et al. (2006) indicated that deep root development in upland rice is genetically determined, and that a nodal root growth angle (also Θ) can serve as a useful tool for rough estimation of vertical root distribution. Therefore, identification of the genetic factors controlling growth angles of roots at the seedling stage is an important key to predicting root system architecture in cereals.
Cereals have two types of roots, the seminal and the nodal. Seminal roots emerge from the germinal embryonic hypocotyls, and nodal roots emerge from the coleoptile nodes at the seedling stage. Detailed studies of gravitropic response of the seminal and nodal roots of wheat were conducted by Oyanagi et al. (1995), Nakamoto and Oyanagi (1994), and Oyanagi (1994). Using agar medium, Oyanagi (1994) found a great deal of variation (range: 4–64°) in the growth angle Θ of the primary seminal roots among Japanese wheat cultivars. He regarded root growth angle as representing the degree of gravitropic response of the seminal root. Nakamoto and Oyanagi (1994) reported that the growth angle of nodal roots is positively correlated with the growth angle of the seminal roots. Based on these findings, they concluded that gravitropic response is the main factor which determines the growth angle of both seminal and nodal roots at the seedling stage. In other words, the root growth angle as evaluated by means of the basket method corresponds to the gravitropic response of the roots.
Root growth angle may be influenced by other environmental conditions such as temperature on the root growth angle, Oyanagi et al. (1993) observed that the effect of temperature was not significant within a range from 10° to 25°. The effect of water availability, on the other hand, was significant: gravitropic response was significantly stimulated when the water potential decreased. This means that wheat roots tend to grow deep into the soil when they sense water stress. Oyanagi et al. (1995) reported that the intensity of hydrotropism in wheat roots and that of gravitropism were independent. These results indicate that genotypic differences in gravitropism and hydrotropism were governed by different genetic factors. Recently, several studies of gravitropism and hydrotropism in plant roots (Morita 2010; Takahashi et al. 2009) have indicated that gravitropic and hydrotropic responses are governed by major genes, and that genotypic differences in the responses of roots to gravity and humidity primarily affect the root growth direction. Thus, these two tropisms play important roles in the establishment of root system architecture. In crops, however, genetic factors contributing to gravitropism and hydrotropism have not been clarified.
Several QTL analyses have been conducted to elucidate the genetic factors underlying root system architecture. These have revealed that the genetic improvement of root system architecture is necessary for improving water- and nutrient-use efficiency of crops or for enhancing their productivity under abiotic stress or suboptimal soil conditions (Dorlodot et al. 2007). Thus, identifying the QTLs that contribute to a plant’s responses to gravity and humidity is an important step in elucidating the effects of gravitropism and hydrotropism on root system architecture. In rice, Uga et al. (2011) evaluated the deep rooting of rice in terms of the proportion of total roots that elongated through the basket bottom (50–90°) and successfully identified a QTL for deep rooting in rice, Dro1. In this study, we conducted two experiments to identify the genetic factors contributing to the seminal and nodal root growth angles. Firstly, we determined the genotypic differences in root growth angle among some landraces originating mainly in various Asian countries to evaluate regional differentiation and genotypic variation in gravitropic response of roots. Secondly, doubled haploid lines (DHLs) were bred from a cross between a line with a shallow root system and a line with a deep root system. Then, QTL analysis for root growth angle in response to gravity and humidity was conducted using the DHLs, to elucidate the genetic factors contributing to adaptive changes in root growth direction.
Materials and methods
Plant materials
Seeds of 194-set genotypes of wheat, including ‘U24’ and ‘Ayahikari’, were germinated, and genetic variance was evaluated among these genotypes in terms of several seminal root traits. Most of the set of 194 genotypes were landraces, mainly originating from various regions of Asia. QTL analyses of several seminal root traits were conducted on 103 DHLs derived from a cross between ‘U24’ and ‘Ayahikari’. ‘U24’ is a local variety with deep root system architecture, originating in the Shinjang district of China. ‘Ayahikari’ is a Japanese commercial variety with shallow root system architecture. The DHLs were developed by crossing maize pollen to the F1 plants (Suenaga and Nakajima 1993) of the cross between ‘U24’ and ‘Ayahikari’ in 2004 and 2006. The set of 194 genotypes were harvested in an experimental field at Kyoto University in 2005–2008. DHLs were harvested at the National Agriculture Research Center for the Kyushu Okinawa Region, Chikugo, Japan, in 2008.
Genetic variation in seminal root traits (Experiment 1)
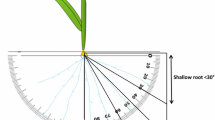
Growth angles of seminal roots and seminal root numbers of the set of 194 genotypes were measured using the basket method (Oyanagi 1994) with slight modification. A plastic meshwork basket (top diameter 16 cm, bottom diameter 8.5 cm, height 6 cm) filled with sand was placed in the top of a 1/5000 Wagner’s pot also filled with sand. Seeds of each line were germinated in Petri dishes with distilled water. Three germinated seeds, each with a 3-mm-long primary seminal root, were transplanted into the center of each basket. Seeds were placed on sand so that each primary root remained horizontal and pointing outward, and seeds were then covered with a 1-cm-thick layer of sand. After transplanting, all pots were placed in a container in which the water level was adjusted to 2 cm, that is, 18 cm below the surface of the pots. Hyponex® was added to the water up to a final concentration of 1/1,000 to supply nutrients. Plants were grown in a greenhouse under controlled conditions at 28°C during daytime and 22°C at night. Seven days after transplanting, baskets were carefully lifted and penetrating roots were observed; their numbers and the location of their penetration were noted. Growth angle of the seminal roots (GA) was determined by measuring the angle between the horizontal and the line connecting the root penetration site and the center of the basket surface (Oyanagi 1994). The raw root growth angle data obtained from each pot were not normally distributed, and a few outliers significantly affected the mean value of GA. Deep root ratio (DRR) was also calculated as the proportion of roots with a growth angle greater than 45°. We chose 45° as the threshold between deep and shallow as 45° is the midpoint between vertical and horizontal base lines. Seminal root number per plant (RN) was calculated by dividing the total number of roots penetrating from the basket mesh by the seedling number. This experiment was conducted according to a randomized block design with two replications (two pots per line).
Construction of a genetic map using doubled haploid lines (Experiment 2)
Total genomic DNAs were extracted from young leaves of 103 DHLs and their parental varieties, ‘Ayahikari’ and ‘U24’, according to the CTAB method with slight modification. A total of 827 simple sequence repeat (SSR) markers (Somers et al. 2004) were examined for polymorphism between ‘U24’ and ‘Ayahikari’. SSR marker analysis was conducted according to the following method. The 12.5-μl PCR reaction mixture contained 25 ng of template DNA, 0.2 mM dNTP, and 6 pM of each primer in EX-Taq PCR buffer containing 0.15 mM MgCl, 3% DMSO, 1 M betain and 0.5 U EX-Taq polymerase (TaKaRa, Shiga, Japan). PCR conditions were as follows: 5 min at 98°C, followed by 40 cycles of 30 s each at 98°C, 30 s at a temperature between 50°C and 65°C depending on the primer combination, and 30 s at 72°C. The last step was incubation at 72°C for 10 min. The PCR products were detected using a capillary gel-electrophoresis system (HDA-GT12; eGene;, Irvine, CA, USA) with a GCK-5000 gel cartridge. Chromosome assignment of each SSR marker was determined on the basis of the molecular linkage map of wheat (Somers et al. 2004). Based on the results of SSR marker analysis, we constructed a genetic linkage map for the 103 DHLs consisting of 281 SSR markers using Antmap v.1.2 (Iwata and Ninomiya 2006, http://lbm.ab.a.u-tokyo.ac.jp/∼iwata/antmap/) and MapMaker/Exp v.3.0 (Lincoln et al. 1993). In applying MapMaker/Exp to the analysis for doubled-haploid lines, we employed ‘f2 backcross’ mode to determine the linkage groups and the marker orders in each linkage group. The maximum recombination value of 0.3 was used to determine linkage between two markers. The frequency of observed recombination between two markers was converted to genetic distance using the map function described by Kosambi (1944).
Phenotyping of the DHLs and QTL analysis for root characteristics (Experiment 3)
GA, RN. and DRR were measured according to the basket method described in Exp. 1 for the 103 DHLs derived from the cross between ‘Ayahikari’ and ‘U24’, using a randomized block design with three replications (three pots per line). To evaluate the effects of hydrotropic response on root growth, we also observed the response of DHLs to humidity. Following Takahashi et al. (2002) and Oyanagi et al. (1995), a humidity gradient was made between a saturated solution of a salt and a wet substrate placed in a closed chamber. When root tips sensed a humidity gradient, they changed their growth direction to grow toward the wet area. We established humidity gradients inside acrylic jars, 9 cm in diameter and 15.5 cm in height, and the experiment was performed as follows: 1% agar was made in a small plastic quadrangular box (W × D × H = 1.5 cm × 1.0 cm × 9.0 cm) and the bottom of each plastic box (2 cm high) was removed to expose the agar prism. A germinated wheat seed with a straight primary seminal root was placed on the surface of the agar so that the root tip was about 1–2 mm from the agar edge. We mounted each seedling to its agar plot by fixing the endosperms with a pin, far enough from the embryo to avoid any damage to it. The top of the plastic box containing the agar block was firmly attached to the inner side of the lid of one of the acrylic jars described above using doubled-sided tape. To establish a humidity gradient inside each jar, 100 ml of saturated K2CO3 solution was placed in the jar bottom. The plastic box and germinated seed were then placed inside the jar with the primary root was positioned vertically, and the jar lid was firmly closed (Fig. 1). The distance between the agar edge and the saturated solution was 4.5 cm. After their lids were firmly closed, the jars were kept at 25°C for 10 h. At the start of the experiment, all root tips were facing the agar surface. When they elongated beyond the edge of the agar, however, many were able to sense the humidity gradient and shifted their growth direction toward the agar. If they could not sense the gradient of humidity, they kept growing vertically. Thus, the angle between the vertical and the line connecting the root tip of each seed and the edge of its agar was measured to determine the response to humidity. The average angle of four seeds was used to estimate the hydrotropic response (HR) of each DHL. In addition to HR, elongation rate (ER) of the primary seminal root was estimated by measuring the root length 36 h after the germination.
The QTL analysis was conducted with WinQTL Cartographer v.2.5 (Wang et al. 2007), a single marker analysis was initially conducted to identify genetic markers significantly associated with phenotypic traits. Then, composite interval mapping (CIM) was conducted at a walk speed of 2 cM. The results of the permutation test indicated that most thresholds for LOD score were greater than 3.5. It is therefore possible that most potential QTLs are undetectable at this threshold. Accordingly, we also examined minor QTLs with LOD score values greater than 2.5.
Results
Genetic variation in seminal root traits
ANOVA applied to our data indicated that there was significant genetic variation among Asian wheat landraces in terms of GA, DRR, and RN (Table 1). Values of GA, DRR and RN ranged from 25° to 60°, 10 to 100%, and 2 to 9, respectively (Fig. 2). While ‘Ayahikari’ exhibited very low values in all three traits, ‘U24’ exhibited very large values. Genotypes were classified into four groups according to their origin: Eastern Asia, Central Asia, Middle East, and Europe (Table 2). Spring-type wheat lines cultivated in eastern Asian countries, including Japan and Korea, exhibited smaller GA and DRR values compared to lines originating in other regions. Growth habit can also affect root architecture: in East Asian and European lines, for example, winter-type wheat tends to exhibit higher GA and DRR values compared to spring-type wheat (Table 2).
Construction of a genetic linkage map
We examined 827 SSR markers for polymorphism between the parental varieties, and from among them we selected 281 polymorphic markers. Then, we constructed a genetic linkage map for DHLs consisting of 276 markers comprising 36 linkage groups (supplementary data). There were 5 markers unlinked to any other markers and 15 gaps in our map. The total map coverage was estimated to be greater than 70% for most wheat chromosomes through comparison with previously constructed linkage maps (Somers et al. 2004). Coverage rates for chromosomes 1D, 4D, 5A, 6A, and 7D were exceptionally low, around 50%, mainly due to the presence of unfilled gaps. We were not able to find either a polymorphic SSR marker to fill up these gaps or a large part of chromosome 5A. The average distance between markers was 9.5 cM.
Phenotyping of the DHLs and QTL analysis for root traits
The frequency distributions of GA, DRR and NR in the DHLs are given in Fig. 2. For all three traits, the DH population exhibited broad and continuous distribution of values ranging between the values of the parental varieties, nearly comparable to the distribution observed among Asian landraces. This indicates that these traits are controlled by multiple loci and that ‘U24’ and ‘Ayahikari’ are representative varieties with deep and shallow root architecture, respectively. Results of ANOVA for root traits indicated that there were significant differences among the DHLs in GA, DRR, and NR, and that estimated heritability of those traits were 0.43, 0.40, and 0.42, respectively.
As there were several gaps in our map, we conducted single marker analysis in addition to CIM analysis to detect QTLs. All the chromosome regions detected through single marker analysis were also detected as QTLs through CIM analysis (Fig. 3). In the CIM analysis, we detected a single QTL controlling RN (qRN) and two QTLs controlling DRR (qDRR-1, qDRR-2), but we could not detect a significant QTL (LOD > 2.5) controlling GA (Table 3). qRN was detected on chromosome 5A, and qDRR-1 and qDRR-2 were detected on chromosomes 1B and 5D, respectively. Each QTL explained 9–11% of the total variation. We also detected QTLs controlling HR and ER of the primary seminal root. The two QTLs controlling HR were detected on 1A (qHR-1) and 2B (qHR-2), clearly indicating that observed DRR is independent from the hydrotropic response of the seminal roots. Finally, the two QTLs controlling ER were detected on 5D (qER-1) and 7D (qER-2).
Discussion
In agreement with previous reports, we have confirmed the presence of significant genotypic differences in root traits among our set of 194 genotypes mainly originating in Asian countries. Oyanagi (1994) have found that wheat varieties bred for western Japan tend to have shallower root systems than varieties bred for eastern Japan, and explained this regional difference as an adaptation to the more abundant soil moisture in western Japan. Thorup-Kristensen et al. (2009) have suggested that the deeper rooting of winter-type wheat enables more effective usage of N in the lower levels of the soil due to N leaching. Manschadi et al. (2008) have found that drought-tolerant varieties tend to have deeper root systems compared to susceptible varieties. These results illustrate the close relationship between root system architecture and soil environmental conditions. The shallow root architecture specific to the East Asian spring varieties was also observed in our results. This might be an adaptation to the high moisture content of the soil due to the Asian monsoon. We also found that winter-type varieties exhibited deeper root architecture than spring-type varieties. In wheat, the spring habit is controlled by Vrn genes, and Vrn-D4 is located on chromosome 5D, where qDRR-2 is also located. The Vrn-D4 locus is located near the centrometric region (Yoshida et al. 2010), however, while qDRR-2 is located on the long arm of chromosome 5D. Therefore, any association between growth habits and the direction of root growth cannot be explained by their linkage. Most of the genotypes tested in the present study are landraces collected from several Asian countries and are commonly cultivated under low N conditions. In this condition, there were very small amounts of N presented in deep layers of the soil. Thus, the deep rooting of winter wheat is not an effective way to access N as has been suggested by Thorup-Kristensen et al. (2009). In our study, however, we were not able to determine the reason why winter-type varieties exhibited deeper root system architecture than spring-type varieties.
In our study, we successfully identified two QTLs for DRR on chromosomes 1B and 5D. Given that the degree of variation in DRR observed among DHLs was nearly comparable to that observed among Asian landraces, the combined effects of these two QTLs could explain most of the variation in DRR among wheat varieties. These two QTLs are assumed to control the gravitropic response of the seminal root of wheat (Oyanagi 1994). It is worth noting that the two gravitropism-related QTLs we have identified here are not located on homoeologous chromosomes. This indicates that there are multiple functionally different genetic factors controlling gravitropism in wheat roots. Uga et al. (2011) found a single major gene, Dro1, for deep rooting in rice. Dro1 is located on the long arm of rice chromosome 9. Comparative genetics studies in cereals (Gale and Devos 1998) have indicated a syntenic relationship between rice chromosome 9 and wheat homoeologous group 5. In addition, a QTL related to root angle of maize has been reported to be located on maize chromosome 7 (Omori and Mano 2007), which has a syntenic relationship with rice chromosome 9 L. Uga et al. (2011) have speculated that a homoeologous relationship exists between Dro1 and the QTL found on maize chromosome 7. As the cloning of Dro1 has already been completed (Uga, personal communication), we will be able to confirm the relationships between Dro1 and qDRR-2, which is located on chromosome 5D, which is related to the gravitropism of wheat. It is therefore possible that a similar regulation system for gravitropism of the roots is distributed widely among cereals. Sharma et al. (2011) have identified QTLs for shallow root weight and deep root weight on the short arm of wheat chromosome 1B (1BS) in a comparison of several recombinants between the short arm of rye chromosome (1RS) and 1BS. Though qDRR-1 is assumed to be located on the long arm of chromosome 1B, further analysis should be conducted to clarify the relationship between qDRR-1 and the previously found QTLs related to deep rooting on chromosome 1B.
We also detected two QTLs related to the hydrotropic response of the primary seminal root on chromosomes 1A and 2B. Like the gravitropism-related QTLs, these two hydrotropism-related QTLs are not located on homoeologous chromosomes. To the best of our knowledge, this is the first report of QTLs related to hydrotropism in crops. Recently, a molecular mechanism underlying hydrotropism in plant roots was partly disclosed in Arabidopsis using the mutant genes miz1 and miz2 (Miyazawa et al. 2009). Though a gene in a monocot cannot be said to have a syntenic relationship with a gene in a dicot, functional analyses of MIZ1 and MIZ2 will be helpful to clarify the regulation system of hydrotropism in wheat. MIZ1 encodes a protein with a MIZ domain of unknown function. The MIZ domain also exists in the genomes of monocots and mosses, but not in the genomes of green algae, red algae, or cyanobacteria. Miyazawa et al. (2009) speculated that the MIZ domain might have played an important role in the initial development of terrestrial plants. Although MIZ2 encodes a GNOM transporter, which is required for proper localization of the auxin efflux, Miyazawa et al. (2009) assumed that MIZ2 plays some undiscovered role in hydrotropism other than its role in auxin polar transport. Our results clearly indicate that gravitropic response and hydrotropic response are controlled by genetically independent factors, as speculated by Oyanagi et al. (1995).
The fact that gravitropism interacts with hydrotropism and usually overwhelms hydrotropic response usually makes it difficult to study hydrotropism in roots. In wheat, however, hydrotropism can easily be evaluated even in the presence of gravity. ‘U24’, one of the parental lines of our DHLs, is highly responsive to both gravity and humidity. Therefore, ‘U24’ harbors an ideal genotype for finding water in the soil. When water resources are limited in the upper part of the soil, the roots of ‘U24’ become highly responsive to gravity and spread into deeper soil. Manschadi et al. (2006) have reported that the drought-tolerant CIMMYT wheat line ‘SeriM82’ exhibited better yielding ability than the Australian wheat line ‘Hartog’ under water shortage conditions. They showed that the narrow root system architecture of ‘SeriM82’ is beneficial for the efficient extraction of soil moisture, and that the architecture of the entire root system is influenced by the traits of the seminal root. In addition, they found that the drought-tolerant line allocated less root growth laterally and produced more root length at deep layers. Using gel chambers at the seedling stage, Bengough et al. (2004) observed a narrower angular spread of the seminal roots of wild-type barley when compared with modern cultivars. They conceded that wild-type barley has been selected to survive in drought conditions by obtaining water from deep soil layers. These results support the idea that deep rooting is beneficial to obtaining water from deep soil layers. In rice (Uga et al. 2011; Steele et al. 2006), sorghum (Tsuji et al. 2005), and maize (Hund et al. 2009; Landi et al. 2007), several studies have reported positive associations between yield and root architecture under drought conditions. However, QTL analyses for drought tolerance in wheat have not revealed effective QTLs related to root system architecture. Fleury et al. (2010) have proposed that different types of drought conditions require different types of root system architecture to optimize the extraction of water from the soil.
In the present study, we detected QTLs for gravitropism and hydrotropism of the wheat root. Using these QTLs, we will be able to construct four different combinations of tropisms: gravitropism only, hydrotropism only, both gravitropism and hydrotropism, and neither of those tropisms. This will enable us to evaluate the combination that is most effective in each specific type of drought conditions and to select for that combination. We can also breed isogenic lines for hydrotropism and gravitropism. Henry et al. (2010) suggested that the multilines (genetic mixture) of contrasting root architecture are beneficial to optimize soil resource utilization and to acquire tolerance to drought and low phosphorus stress. Mixed cultivation of different kinds of isogenic lines for the two tropisms will also be beneficial to avoid competition among the plants for water in the soil. To establish efficient marker-aided selection for the two kinds of tropism, we will identify the corresponding genes through fine mapping.
References
Bengough AG, Gordon DC, Al-Menaie H, Ellis RP, Allan DL, Keith R, Thomas WTB, Forster BP (2004) Gel observation chamber for rapid screening of root traits in cereal seedlings. Plant Soil 262:63–70. doi:10.1023/B:PLSO.0000037029.82618.27
Dorlodot SD, Forster B, Pagés L, Price A, Tuberosa R, Draye X (2007) Root system architecture: opportunities and constraints for genetic improvement of crops. Trends Plant Sci 12:474–481. doi:10.1016/j.tplants.2007.08.012
Fleury D, Jefferies S, Kuchel H, Langridge P (2010) Genetic and genomic tools to improve drought tolerance in wheat. J Exp Bot 61:3211–3222. doi:10.1093/jxb/erq152
Gale MD, Devos KM (1998) Comparative genetics in the grasses. Proc Natl Acad Sci USA 95:1971–1974
Henry A, Rosas JC, Beaver JS, Lynch JP (2010) Multiple stress response and belowground competition in multilines of common bean (Phaseolus vulgaris L.). Field Crop Res 117:209–218. doi:10.1016/j.fcr.2010.03.004
Hund A, Ruta N, Liedgens M (2009) Rooting depth and water use efficiency of tropical maize inbred lines, differing in drought tolerance. Plant Soil 318:311–325. doi:10.1007/s11104-008-9843-6
Iwata H, Ninomiya S (2006) AntMap: Constructing genetic linkage maps using an ant colony optimization algorithm. Breed Sci 56:371–377. doi:10.1270/jsbbs.56.371
Kato Y, Abe J, Kamoshita A, Yamagishi J (2006) Genotypic variation in root growth angle in rice (Oryza sativa L.) and its association with deep root development in upland fields with different water regimes. Plant Soil 287:117–129. doi:10.1007/s11104-006-9008-4
Kosambi DD (1944) The estimation of map distances from recombination values. Ann Eugen 12:172–175
Landi P, Sanguineti MC, Liu C, Li Y, Wang TY, Giuliani S, Bellotti M, Salvi S, Tuberosa R (2007) Root-ABA1 QTL affects root lodging, grain yield, and other agronomic traits in maize grown under well-watered and water-stressed conditions. J Exp Bot 58:319–326. doi:10.1093/jxb/erl161
Lincoln SE, Daly MJ, Lander ES (1993) Constructing genetic linkage maps with MAPMAKER, version 3: a tutorial and reference manual, 3rd edn. Whitehead Institute for Biomedical Research, Cambridge
Manschadi AM, Christopher JT, deVoil P, Hammer GL (2006) The role of root architectural traits in adaptation of wheat to water-limited environments. Funct Plant Bio 33:823–837. doi:10.1071/FP06055
Manschadi AA, Hammer GL, Christopher JT, deVoil P (2008) Genotypic variation in seedling root architectural traits and implications for drought adaptation in wheat (Triticum aestivum L.). Plant Soil 303:115–129. doi:10.1007/s11104-007-9492-1
Miyazawa Y, Takahashi A, Kobayashi A, Kaneyasu T, Fujii N, Takahashi H (2009) The GNOM-mediated vesicular trafficking plays an essential role in hydrotropism of Arabidopsis roots. Plant Physiol 149:835–840. doi:10.1104/pp.108.131003
Morita MT (2010) Directional gravity sensing in gravitropism. Annu Rev Plant Biol 61:705–720. doi:10.1146/annurev.arplant.043008.092042
Nakamoto T, Oyanagi A (1994) The direction of growth of seminal roots of Triticum aestivum L. and experimental modification thereof. Ann Bot 73:363–367
Omori F, Mano Y (2007) QTL mapping of root angle in F2 populations from maize ‘B73’ × teosinte ‘Zea luxurians’. Plant Root 1:57–65. doi:10.3117/plantroot.1.57
Osmont KS, Sibout R, Hardtke CS (2007) Hidden branches: developments in root system architecture. Annu Rev Plant Biol 58:93–113. doi:10.1146/annurev.arplant.58.032806.104006
Oyanagi A (1994) Gravitropic response growth angle and vertical distribution of roots of wheat (Triticum aestivum L.). Plant Soil 165:323–326
Oyanagi A, Nakomoto T, Morita S (1993) The gravitropic response of roots and the shaping of the root system in cereal plants. Environ Exp Bot 33:144–158
Oyanagi A, Takahashi H, Suge H (1995) Interactions between hydrotropism and gravitropism in the primary seminal roots of Triticum aestivum L. Ann Bot (Lond) 75:229–235
Sharma S, Xu S, Ehdaie B, Hoops A, Close TJ, Lukaszewski AJ, Waines JG (2011) Dissection of QTL effects for root traits using a chromosome arm-specific mapping population in bread wheat. Theor Appl Genet 122:759–769. doi:10.1007/s00122-010-1484-5
Somers DJ, Isaac P, Edwards K (2004) A high-density microsatellite consensus map for bread wheat. (Triticum aestivum L.). Theor Appl Genet 109:1105–1114. doi:10.1007/s00122-004-1740-7
Steele KA, Price AH, Shashidhar HE, Witcombe JR (2006) Marker-assisted selection to introgress rice QTLs controlling root traits into an Indian upland rice variety. Theor Appl Genet 112:208–221. doi:10.1007/s00122-005-0110-4
Suenaga K, Nakajima K (1993) Variation in doubled haploid plants of wheat obtained through wheat (Triticum aestivum) × Maize (Zea mays) crosses. Plant Breed 111:120–124
Takahashi N, Goto N, Okada K, Takahashi H (2002) Hydrotropism in abscisic acid, wavy, and gravitropic mutants of Arabidopsis thaliana. Planta 216:203–211. doi:10.1007/s00425-002-0840-3
Takahashi H, Miyazawa Y, Fujii N (2009) Hormonal interactions during root tropic growth: hydrotropism versus gravitropism. Plant Mol Biol 69:489–502. doi:10.1007/s11103-008-9438-x
Thorup-Kristensen K, Cortasa MS, Loges R (2009) Winter wheat roots grow twice as deep as spring wheat roots, is this important for N uptake and N leaching losses? Plant Soil 322:101–114. doi:10.1007/s11104-009-9898-z
Tsuji W, Inanaga S, Araki H, Morita S, An P, Sonobe K (2005) Development and distribution of root system in two grain Sorghum cultivars originated from Sudan under drought stress. Plant Prod Sci 8:553–562. doi:10.1626/pps.8.553
Uga Y, Okuno K, Yano M (2011) Dro1, a major QTL involved in deep rooting of rice under upland field conditions. J Exp Bot. doi:10.1093/jxb/erq429
Wang S, Basten CJ, Zeng ZB (2007) Windows QTL Cartographer 2.5. Department of Statistics, North Carolina State University, Raleigh, NC. http://statgenncsuedu/qtlcart/WQTLCarthtml
Yoshida T, Nishida H, Zhu J, Nitcher R, Distelfeld A, Akashi Y, Kato K, Dubcovsky J (2010) Vrn-D4 is a vernalization gene located on the centromeric region of chromosome 5D in hexaploid wheat. Theor Appl Genet 120:543–552. doi:10.1007/s00122-009-1174-3
Acknowledgment
This study was supported in part by a grant from the National Bioresource Project of the Japanese Ministry of Education, Culture, Sports, Science and Technology.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Matthias Wissuwa.
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Hamada, A., Nitta, M., Nasuda, S. et al. Novel QTLs for growth angle of seminal roots in wheat (Triticum aestivum L.). Plant Soil 354, 395–405 (2012). https://doi.org/10.1007/s11104-011-1075-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-011-1075-5