ABSTRACT
Purpose
In order to improve formulation of targeting chemotherapy, cisplatin-loaded magnetic solid lipid nanoparticles (MSLNs) were prepared. In present study, the deliberate loading of Fe3O4 magnetic nanoparticles (MNs) into cisplatin SLNs was developed.
Methods
SLNs were produced by film scattering ultrasonic technique. The effects of two different loading procedures of MNs on the microstructure and physicochemical properties of MSLNs were investigated by transmission electron microscopy (TEM), zetasizer, infrared spectroscopy (IR), and fluorescence spectroscopy. In vitro drug release and cytotoxicity against human cervical carcinoma SiHa cells, in vivo tumor cell uptake and target tissue distribution of MSLNs under external magnetic field were investigated.
Results
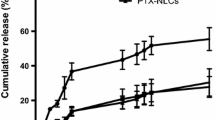
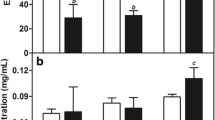
The encapsulation efficiency of cisplatin and the content of MNs in procedure I SLNs were 69.20 ± 4.5% and 2.16 ± 0.53 mg/mL, respectively, which were higher than those of procedure II MSLNs. In procedure I, the MNs, which were combined with lipids during film formation, distributed in the middle of the lipid layer in SLNs. Differently, in procedure II, the MNs and cisplatin were contained in an interior compartment in SLNs, resulting from mixing with drugs during hydration of lipid film. The procedure I MSLNs had higher cytotoxicity than procedure II MSLNs or free cisplatin. With in vivo intratumoral administration, cisplatin concentration in the tumor tissue was maintained at higher level for MSLNs than that for free cisplatin, especially under external magnetic field.
Conclusions
Procedure I, the developed deliberate MNs loading method, was superior over procedure II in cisplatin encapsulation efficiency, MNs content and cell cytotoxicity.






Similar content being viewed by others
Abbreviations
- AAS:
-
Graphite furnace atomic absorption spectrometer
- DMSO:
-
Dimethyl sulfoxide
- EE:
-
Encapsulation efficiency
- F-68:
-
Poloxamer 188
- FBS:
-
Fetal bovine serum
- FT-IR:
-
Fourier transform infrared spectroscopy
- GMS:
-
Glycerol monostearate
- HSPC:
-
Hydrogenated soybean lecithin
- MNs:
-
Magnetic nanoparticles
- MSLNs:
-
Magnetic solid lipid nanoparticles
- MTT:
-
3-[4,5-Dimethylthiazol-2-yl]-2,5-diphenyl-tetrazolium bromide
- PBS:
-
Phosphate-buffered saline
- PDI:
-
Polydispersity index
- SLNs:
-
Solid lipid nanoparticles
- TEM:
-
Transmission electron microscopy
REFERENCES
Ali BH, Al-Moundhri MS. Agents ameliorating or augmenting the nephro-toxicity of cisplatin and other platinum compounds: a review of somerecent research. Food Chem Toxicol. 2006;44(8):1173–83.
Marchion DC, Xiong Y, Chen N, Bicaku E, Stickles XB, et al. The BCL2 antagonist of cell death pathway influences endometrial cancer cell sensitivity to cisplatin. Gynecol Oncol. 2012;124(1):119–24.
Mitsudomi T, Morita S, Yatabe Y, Negoro S, Okamoto I, Tsurutani J, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring muta-tions of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. 2010;11:121–8.
Kuang Y, Liu J, Liu ZL, Zhuo RX. Cholesterol based anionic long-circulating cisplatin liposomes with reduced renal toxicity. Biomaterials. 2012;33(5):1596–56.
Qu J, Li X, Wang J, Mi WJ, Xie KL, Qiu JH. Inhalation of hydrogen gas attenuates cisplatin-induced ototoxicity via reducing oxidative stress. Int J Pediatr Otorhinolaryngol. 2012;76(1):111–5.
Li H, Zhao X, Ma Y, Zhai G, Li L, Lou H. Enhancement of gastrointestinal absorption of quercitin by solid lipid nanoparticles. J Control Release. 2009;133(3):238–44.
Ying XY, Cui D, Yu L, Du YZ. Solid lipid nanoparticles modified with chitosan oligosaccharides for the controlled release of doxorubicin. Carbohydr Polym. 2011;84(4):1357–64.
Santander-Ortega MJ, Lozano-Lopez MV, Bastos-Gonzalez D, Peula-Garcia JM, Ortega-Vinuesa JL. Novel core-shell lipid chitosan and lipid-poloxamer nanocapsules: stability by hydration forces. Colloid Polym Sci. 2010;288(2):159–72.
Wu LF, Tang C, Yin CH. Folate-mediated solid–liquid lipid nanoparticles for paclitaxel-coated poly (ethylene glycol). Drug Dev Ind Pharm. 2010;36(4):439–48.
Zhang WL, Liu JP, Li SC, Chen MY, Liu H. Preparation and evaluation of stealth tashinone IIA-loaded solid lipid nanoparticles: influence of Poloxamer 188 coating on phagocytic uptake. J Microencapsul. 2008;25(3):203–9.
Nobuto H, Sugita T, Kubo T, Shimose S, Yasunaga Y, Murakami T, et al. Evaluation of systemic chemotherapy with magnetic liposomal doxorubicin and a dipole external electromagnet. Int J Cancer. 2004;109(4):627–35.
Zhang JQ, Zhang ZR, Yang H, Tan QY, Qin SR, Qiu XL. Lyophilized paclitaxel magnetoliposomes as a potential drug delivery system for breast carcinoma via parenteral administration: in vitro and in vivo studies. Pharm Res. 2005;22(4):573–83.
Cavalli R, Caputo O, Gasco MR. Solid lipospheres of doxorubicin and idarubicin. Int J Pharm. 1993;89(1):9–12.
Miglietta A, Cavallib R, Boccaa C, Bocca C, Gabriel L, Gasco MR. Cellular uptake and cytotoxicity of solid lipid nanospheres (SLN) incorporating doxorubicin or paclitaxel. Int J Pharm. 2000;210(1–2):61–7.
Uagio E, Cavalli R, Gasco MR. Incorporation of cyclosporin A in solid lipid nanoparticles (SLN). Int J Pharm. 2002;241(2):341–4.
Westesen K, Siekmann B. Investigation of the gel formation of phospholipid-stabilized solid lipid nanoparticles. Int J Pharm. 1997;151(1):35–45.
Zhao S, Yang CQ, Yan JW, Wang J. A novel solvethermal method for the preparation of magnetic monodisperse Fe3O4 nanoparticles II: High-surface-activity ferrihydrite used as precursor. Mater Res Bull. 2013;48(10):4385–9.
Manjunath K, Venkateswarlu V. Pharmacokinetics, tissue distribution and bioavailability of clozapine solid lipid nanoparticles after intravenous and intraduodenal administration. J Control Release. 2005;107(2):215–28.
Liu SL, Zhang LN, Zhou JP, Wu RX. Structure and properties of cellulose/Fe2O3nanocomposite fibers spun via an effective pathway. J Phys Chem C. 2008;112(12):4538–44.
Wang L, Yang CQ, Wang J. Effects of loading procedures of magnetic nanoparticles on the structure and physicochemical properties of cisplatin magnetic liposomes. J Microencapsul. 2012;29(8):781–9.
Jenning V, Mäder K, Gohla SH. Solid Lipid Nanoparticles (SLN) based on binary mixtures of liquid and solid lipids: a (1)H-NMR study. Int J Pharm. 2000;205(1–2):15–21.
García-Fuentes M, Torres D, Alonso MJ. Design of lipid nanoparticles for the oral delivery of hydrophilic macromolecules. Colloid Surf B. 2002;27(2–3):159–68.
Mehnert W, Mäder K. Solid lipid nanoparticles-production, characterization and applications. Adv Drug Deliv Rev. 2001;47(2–3):165–96.
Schwarz C, Mehnert W, Lucks JS, Miiller RH. Solid Lipid Nanoparticles (SLN) for controlled drug delivery. I. Production, characterization and sterilization. J Control Release. 1994;30:83–96.
Bloch K, Bangham AD, Scherphof GL, Kennedy EP, Waite M, Hostetler KY. Lipids and mombranes: past. Presint and future. Amsterdam: Elsever; 1986. p. 336.
Wang W, Li LM, Li ZL, Xi SG. The molecular mechanisms of interaction between rare earth irons and DPPC liposome. Spectrosc Spectr Anal. 1993;13(5):51–4.
Neumann MG, Schmitt CC, Iamazaki ET. A Fuorescence study of the interactions between sodium alginate and surfactants. Permissions Repr. 2003;338(10):1109–13.
Thakkar VT, Shah PA, Soni TG, Parmar MY, Gohel MC, Gandhi TR. Goodness-of-fit model-dependent approach for release kinetics of levofloxacin hemihydrates floating tablet. Dissolut Technol. 2009;16(1):35–9.
Yokoyama M, Okano T, Sakurai Y, Suwa S, Kataoka K. Introduction of cisplatin into polymeric micelle. J Control Rel. 1996;39:351–6.
Hwang TL, Lee WR, Hua SC, Fang JY. Cisplatin encapsulated in phosphatidylethanolamine liposomes enhances the in vitro cytotoxicity and in vivo intratumor drug accumulation against melanomas. J Dermatol Sci. 2007;46:11–20.
Vasir JK, Reddy MK, Labhasetwar VD. Nanosystems in drug targeting: opportunities and challenges. Curr Nanosci. 2005;1(1):47–64.
ACKNOWLEDGMENTS AND DISCLOSURES
The financial support for this work by the Hebei Provincial Natural Science Fund of China (H2013206040, 2008001072) is greatly acknowledged.
Author information
Authors and Affiliations
Corresponding authors
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 138 kb)
Rights and permissions
About this article
Cite this article
Zhao, S., Zhang, Y., Han, Y. et al. Preparation and Characterization of Cisplatin Magnetic Solid Lipid Nanoparticles (MSLNs): Effects of Loading Procedures of Fe3O4 Nanoparticles. Pharm Res 32, 482–491 (2015). https://doi.org/10.1007/s11095-014-1476-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-014-1476-2