No Heading
Purpose.
The study reports in vitro and biological evaluation of lyophilized negatively charged paclitaxel magnetic liposomes as a potential carrier for breast carcinoma via parenteral administration.
Methods.
Paclitaxel in magnetoliposomes were extracted by centrifugation and quantified by high-performance liquid chromatography (HPLC). Biological properties were studied using pharmacokinetics, in vivo distribution and cytotoxicity assays, as well as a mouse model of EMT-6 breast cancer.
Methods.
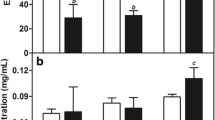
Pharmacokinetic studies showed that encapsulation of paclitaxel in magnetoliposomes produced marked difference over the drug in Cremophor EL/ethanol pharmacokinetics, with an increased t1/2β 19.37 h against 4.11 h. For in vivo distribution, paclitaxel concentration of lyophilized magnetoliposomes in the tumor was much higher than that of lyophilized conventional liposomes or Cremophor EL/ethanol, whereas in heart it was much lower than the latter two formulations via s.c. and i.v. administration. Lyophilized paclitaxel magnetic liposomes showed more potency on the therapy of breast cancer than other formulations via s.c. and i.p. administration.
Conclusions.
The current study demonstrates that paclitaxel magnetoliposomes can effectively be delivered to tumor and exert a significant anticancer activity with fewer side effects in the xenograft model.
Similar content being viewed by others
References
1. E. C. K. Rowinsky, L. A. Cazenave, and R. C. Donehower. Taxol: a novel investigational antimicrotubule agent. J. Natl. Cancer Inst. 82:1247–1259 (1990).
2. E. K. Rowinsky, N. Onetto, R. M. Canetta, and S. G. Arbuck. Taxol: the first of the taxanes, an important new class of antitumor agents. Semin. Oncol. 19:646–662 (1992).
3. R. B. Weiss, R. C. Donehower, P. H. Wiernik, T. Ohnuma, R. J. Gralla, and D. Trump. Hypersensitivity reactions from taxol. J. Clin. Oncol. 8:1263–1268 (1990).
4. S. B. Horwitz. Mechanism of action of taxol. Trends Pharmacol. Sci. 13:134–136 (1992).
5. E. K. Rowinsky. The development and clinical utility of the taxane class of antimicrotubule chemotherapy agents. Annu. Rev. Med. 48:353–374 (1997).
6. G. E. Capri, E. Tarenzi, F. Fulfaro, and L. Gianni. The role of taxanes in the treatment of breast cancer. Semin. Oncol. 23 (1. Suppl. 2):68–75 (1996).
7. R. B. Weiss, R. C. Donehower, P. H. Wiernik, T. Ohnuma, R. J. Gralla, and D. Trump. Hypersensitivity reactions from taxol. J. Clin. Oncol. 8:1263–1268 (1990).
8. R. T. Dorr. Pharmacology and toxicology of Cremophor EL diluent. Ann. Pharmacother. 28:S11–S14 (1994).
9. D. D. Lasic. Recent developments in medical applications of liposomes: sterically stabilized liposomes in cancer therapy and gene delivery in vivo J. Control. Release 48:203–222 (1997).
10. M. C. Woodle. Sterically stabilized liposome therapeutics. Adv. Drug Deliv. Rev. 16:249–265 (1997).
11. A. Sharma and R. M. Straubinger. Novel taxol formulations: preparation and characterization of taxol-containing liposomes. Pharm. Res. 11:889–896 (1994).
12. A. Sharma, E. Mayhew, and R. M. Straubinger. Antitumor effect of taxol-containing liposomes in a taxol-resistant murine tumor model. Cancer Res. 53:5877–5881 (1993).
13. R. Kirsh, P. J. Bugleski, and G. Poste. Drug delivery to macrophages for the therapy of cancer and infectious diseases. In: R. L. Juliano (ed.), Biological Approaches to the Controlled Delivery of Drugs, New York Academy of Sciences, New York, 1987, pp. 141–154.
14. G. L. Scherphof. In vivo behavior of liposomes. In: R. L. Juliano (ed.), Targeted Drug Delivery, Springer, Berlin, New York, 1991, pp. 285–300.
15. H. Kiwada, J. Sato, S. Yamada, and Y. Kato. Feasibility of magnetic liposomes as a targeting device for drugs. Chem. Pharm. Bull. (Tokyo) 34:4253–4258 (1986).
16. M. Shinkai, M. Suzuki, S. Iijima, and T. Kobayashi. Antibody-conjugated magneto-liposomes for targeting cancer cells and their application in hyperthermia. Biotechnol Appl Bilchem 21:125–137 (1995).
17. M. Shinkai, M. Yanase, H. Honda, T. Wakabayashi, J. Yoshida, and T. Kobayashi. Intrcellular hyperthermia for cancer using magnetite cationic liposomes: in vitro study. Jpn. J. Cancer Res. 87:1179–1183 (1996).
18. E. Viroonchatapan, M. Ueno, H. Sato, I. Adachi, H. Nagae, K. Tazawa, and I. Horikoshi. Preparation and characterization of dextran-magnetite incorporated thermosensitive liposomes: an on-line flow system for quantifying magnetic responsiveness. Pharm. Res. 12:1176–1183 (1995).
19. T. Kubo, T. Sugita, S. Shimose, Y. Nitta, Y. Ikuta, and T. Murakami. Targeted delivery of anticancer drugs with intravenously administered magnetic liposomes in osteosarcoma-bearing hamsters. Int. J. Oncol. 17:309–315 (2000).
20. J. Sanyog, M. Vivek, S. Paramjit, P. K. Dubey, D. K. Saraf, and S. P. Vyas. RGD-anchored magnetic liposomes for monocytes/neutrophils-mediated brain targeting. Int. J. Pharm. 261:43–55 (2003).
21. S. L. Richheimer, D. M. Tinnermeier, and D. W. Timmons. High-performance liquid chromatographic assay of taxol. Anal. Chem. 64:2323–2333 (1992).
22. J. Q. Zhang, Z. R. Zhang, and H. Luo. Determination of paclitaxel and the entrapment of its formulated preparations by RP-HPLC. West China J. Pharm. Sci. 16:96–97 (2001).
23. T. D. Heath and C. S. Brown. Liposome dependent delivery of N-(phosphonacetyl)-L-aspartic acid to cells in vitro. J. Liposome Res. 1:303–317 (1989–90).
24. C. Fonseca, S. Simoes, and R. Gaspar. Paclitaxel-loaded PLGA nanoparticles: preparation, physicochemical characterization and in vitro anti-tumoral activity. J. Control. Rel. 83:273–286 (2002).
25. Z. H. Ge, H. Li, J. F. Xue, and N. Wang. Determination of taxol in dog plasma, mouse tissue, and urine, feces by HPLC. Chin. J. Pharm. Anal 17:301–304 (1997).
26. M. D. Bethesda. Division of cancer treatment, NCI. Clinical Brochure: Taxol (NSC 125973). National Cancer Institute, Frederick, MA, 1983, pp. 6–12.
27. National Institutes of Health. Principals of Laboratory Animal Care. Publication No.85-23. National Institutes of Health, Bethesda, MD: Revised 1985.
28. A. Sharma, E. Mayhew, L. Bolcsak, C. Cavanaugh, P. Harmon, A. Janoff, and R. J. Bernacki. Activity of paclitaxel liposome formulations against human ovarian tumor xenografts. Int. J. Cancer 71:103–107 (1997).
29. C. Paola, C. Maurizio, B. Paola, A. Silvia, D. Franco, and C. Luigi. Preparation, characterization and properties of sterically stabilized paclitaxel-containing liposomes. J. Control. Rel. 63:19–30 (2000).
30. R. R. C. New. Introduction and preparation of liposomes. In R. R. C. New, (ed.), Liposomes: A Practical Approach, Oxford University Press, Oxford, 1990, pp. 1–104.
31. T. Kubo, T. Sugita, S. Shimose, Y. Nitta, Y. Ikuta, and T. Murakami. Targeted systemic chemotherapy using magnetic liposomes with incorporated Adriamycin for osteosarcoma in hamsters. Int. J. Oncol. 18:121–125 (2001).
32. N. Hiroo, S. Takashin, K. Tadahiko, S. Shoji, Y. Yuji, M. Teruo, and O. Mitsuo. Evaluation of systemic chemotherapy with magnetic liposomal doxorubicin and a dipole external electromagnet. Int. J. Cancer 109:627–635 (2004).
33. K. S. Wu, J. T. Tang, X. Liu, and Q. Zhang. Preparation of magnetoliposomes and its in vivo behavior on ICR mice. Yao Xue Xue Bao 39:288–291 (2004).
34. H. Shigeaki, T. Iwai, I. Akira, M. Kenji, S. Toshio, I. Masafumi, H. Hiroyuki, K. Takeshi, and U. Minoru. Selective hyperthermia using magnetoliposomes to target cervical lymph node metastasis in a rabbit tongue tumor model. Cancer Sci. 94:834–839 (2003).
35. A. Sharma, N. L. Straubinger, and R. M. Straubinger. Modulation of human ovarian tumor cell sensitivity to N-(phosphon-acetyl)-L-aspartate (PALA) by liposome drug carriers. Pharm. Res. 10:1434–1441 (1993).
36. M. Thole, S. Nobmanna, J. Huwyler, A. Bartmann, and G. Fricker. Uptake of cationzied albumin coupled liposomes by cultured porcine brain microvessel endothelial cells and intact brain capillaries. J. Drug Target. 10:337–344 (2002).
37. P. K. Gupta and C. T. Hung. Comparative disposition of adriamycin delivered via magnetic albumin microspheres in presence and absence of magnetic field in rats. Life Sci. 46:471–479 (1990).
38. L. B. Margolis and V. A. Namiot. L. M. Kliukin- cell sorting using magnetoliposomes. Biofizika 28:884–885 (1983).
39. C. A. Alexios, W. Arnold, R. J. Klein, F. G. Parak, P. Hulin, C. Bergmann, W. Erhardt, S. Wagenpfeil, and A. S. Lubbe. Locoregional cancer treatment with magnetic drug targeting. Cancer Res. 60:6641–6648 (2000).
40. A. S. Lubbe, C. Bergemann, W. Huhnt, T. Fricke, H. Riess, J. W. Huhn, and D. Huhn. Preclinical experiences with magnetic drug targeting: tolerance and efficacy. Cancer Res. 56:4694–4701 (1996).
41. A. S. Lubbe, C. Bergemann, H. Riess, F. Schriver, P. Reichardt, K. Mattias, M. Matthias, B. Dorken, F. Herrmann, R. Gurtler, P. Haas, N. Haas, R. Schr, B. Sander, A. J. Lemke, D. Ohlendorf, W. Huhnt, and D. Huhn. Clinical experiences with magnetic drug targeting: a Phase I study with 4’-epidoxorubicin in 14 patients with advanced solid tumors. Cancer Res. 56:4686–4693 (1996).
42. A. Sharma, W. D. Conway, and R. M. Straubinger. Reversed-phase high-performance liquid chromatographic determination of taxol in mouse plasma. J. Chromatogr B. 655:315–319 (1994).
43. M. Babincova, P. Cicmanec, and V. Atlanerova. AC-magnetic field controlled drug release from magnetoliposomes: design of a method for site-specific chemotherapy. Bioelectrochemistry 55:17–19 (2002).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Zhang, J., Zhang, Z., Yang, H. et al. Lyophilized Paclitaxel Magnetoliposomes as a Potential Drug Delivery System for Breast Carcinoma via Parenteral Administration: In Vitro and in Vivo Studies. Pharm Res 22, 573–583 (2005). https://doi.org/10.1007/s11095-005-2496-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-005-2496-8