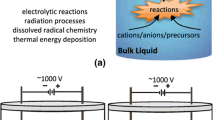
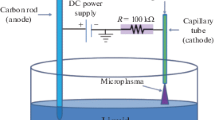
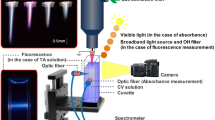
Abstract
In this work, we investigate the production of highly oxidative species in solutions exposed to a self-pulsed corona discharge in air. We examine how the properties of the target solution (pH, conductivity) and the discharge power affect the discharge stability and the production of H2O2. Indigo carmine, a common organic dye, is used as an indicator of oxidative strength and in particular, hydroxyl radical (OH·) production. The observed rate of indigo oxidation in contact with the discharge far exceeds that predicted from reactions based on concentrations of species measured in the bulk solution. The generation of H2O2 and the oxidation of indigo carmine indicate a high concentration of highly oxidizing species such as OH· at the plasma–liquid interface. These results indicate that reactions at the air plasma–liquid interface play a dominant role in species oxidation during direct non-equilibrium atmospheric pressure plasma treatment.










Similar content being viewed by others
Notes
In simple experiments in which methyl red solutions (buffered to pH 7.4) were treated with NEAPP, no color change (indicating a gradient in pH) was observed near the surface or anywhere in solution. Solutions were treated with NEAPP until the buffer was overcome, at which point the color uniformly changed from yellow to red. This is in contrast to experiments performed by Witzke et al. [79] (in the absence of convection), in which spatial gradients in pH were observed at the plasma-liquid interface. This suggests that any gradients in pH that exist are restricted to very near the surface.
The rate at which 1O2 is quenched (reduced to the triplet state) by indigo carmine is 3.2 × 108 M−1 s−1 per the results of Gandra et al. [76]. Moreover, the quenching of 1O2 observed in these experiments was stated to occur through “physical quenching”, or processes that convert 1O2 to 3O2 by reducing the energy state of indigo carmine rather than changing its structure (“chemical quenching”). Oxidation products of indigo carmine were only observed after extended irradiation periods (several hours of 1O2 production).
References
Graves DB (2012) The emerging role of reactive oxygen and nitrogen species in redox biology and some implications for plasma applications to medicine and biology. J Phys D Appl Phys 45(26):263001
von Woedtke T, Metelmann H-R, Weltmann K-D (2014) Clinical plasma medicine: state and perspectives of in vivo application of cold atmospheric plasma. Contrib Plasma Phys 54(2):104–117
Lu X, Laroussi M, Puech V (2012) On atmospheric-pressure non-equilibrium plasma jets and plasma bullets. Plasma Sour Sci Technol 21(3):034005
Alkawareek MY, Algwari QT, Laverty G, Gorman SP, Graham WG, O’Connell D, Gilmore BF (2012) Eradication of Pseudomonas aeruginosa biofilms by atmospheric pressure non-thermal plasma. PLoS ONE 7(8):13–15
Aboubakr HA, Williams P, Gangal U, Youssef MM, El-Sohaimy SAA, Bruggeman PJ, Goyal SM (2015) Virucidal effect of cold atmospheric gaseous plasma on feline calicivirus, a surrogate for human norovirus. Appl Environ Microbiol 81(11):3612–3622
Cahill OJ, Claro T, O’Connor N, Cafolla AA, Stevens NT, Daniels S, Humphreys H (2014) Cold air plasma to decontaminate inanimate surfaces of the hospital environment. Appl Environ Microbiol 80(6):2004–2010
Pavlovich MJ, Clark DS, Graves DB (2014) Quantification of air plasma chemistry for surface disinfection. Plasma Sour Sci Technol 23(6):065036
Brisset J-L, Benstaali B, Moussa D, Fanmoe J, Njoyim-Tamungang E (2011) Acidity control of plasma-chemical oxidation: applications to dye removal, urban waste abatement and microbial inactivation. Plasma Sour Sci Technol 20(3):034021
Jiang B, Zheng J, Qiu S, Wu M, Zhang Q, Yan Z, Xue Q (2014) Review on electrical discharge plasma technology for wastewater remediation. Chem Eng J 236:348–368
Wang T, Ma T, Qu G, Liang D, Hu S (2014) Performance evaluation of hybrid gas-liquid pulse discharge plasma-induced degradation of polyvinyl alcohol-containing wastewater. Plasma Chem Plasma Process 34(5):1115–1127
Mariotti D, Sankaran RM (2010) Microplasmas for nanomaterials synthesis. J Phys D Appl Phys 43(32):323001
Richmonds C, Sankaran RM (2008) Plasma–liquid electrochemistry: rapid synthesis of colloidal metal nanoparticles by microplasma reduction of aqueous cations. Appl Phys Lett 93(13):131501
Bouchard M, Létourneau M, Sarra-Bournet C, Laprise-Pelletier M, Turgeon S, Chevallier P, Lagueux J, Laroche G, Fortin MA (2015) Rapid nucleation of iron oxide nanoclusters in aqueous solution by plasma electrochemistry. Langmuir 31(27):7633–7643
Tanaka H, Mizuno M, Ishikawa K, Nakamura K, Kajiyama H, Kano H, Kikkawa F, Hori M (2011) Plasma-activated medium selectively kills glioblastoma brain tumor cells by down-regulating a survival signaling molecule, AKT kinase. Plasma Med 1(3–4):265–277
Köritzer J, Boxhammer V, Schäfer A, Shimizu T, Klämpfl TG, Li Y-F, Welz C, Schwenk-Zieger S, Morfill GE, Zimmermann JL, Schlegel J (2013) Restoration of sensitivity in chemo-resistant glioma cells by cold atmospheric plasma. PLoS ONE 8(5):e64498
Utsumi F, Kajiyama H, Nakamura K, Tanaka H, Mizuno M, Ishikawa K, Kondo H, Kano H, Hori M, Kikkawa F (2013) Effect of indirect nonequilibrium atmospheric pressure plasma on anti-proliferative activity against chronic chemo-resistant ovarian cancer cells in vitro and in vivo. PLoS ONE 8(12):e81576
Pavlovich MJ, Chang H-W, Sakiyama Y, Clark DS, Graves DB (2013) Ozone correlates with antibacterial effects from indirect air dielectric barrier discharge treatment of water. J Phys D Appl Phys 46(14):145202
Bundscherer L, Wende K, Ottmüller K, Barton A, Schmidt A, Bekeschus S, Hasse S, Weltmann K-D, Masur K, Lindequist U (2013) Impact of non-thermal plasma treatment on MAPK signaling pathways of human immune cell lines. Immunobiology 218(10):1248–1255
Vatansever F, de Melo WCMA, Avci P, Vecchio D, Sadasivam M, Gupta A, Chandran R, Karimi M, Parizotto NA, Yin R, Tegos GP, Hamblin MR (2013) Antimicrobial strategies centered around reactive oxygen species–bactericidal antibiotics, photodynamic therapy, and beyond. FEMS Microbiol Rev 37(6):955–989
Lindsay A, Anderson C, Slikboer E, Shannon S, Graves D (2015) Momentum, heat, and neutral mass transport in convective atmospheric pressure plasma–liquid systems and implications for aqueous targets. J Phys D Appl Phys 48(42):424007
Lukes P, Dolezalova E, Sisrova I, Clupek M (2014) Aqueous-phase chemistry and bactericidal effects from an air discharge plasma in contact with water: evidence for the formation of peroxynitrite through a pseudo-second-order post-discharge reaction of H2O2 and HNO2. Plasma Sour Sci Technol 23(1):015019
Machala Z, Tarabova B, Hensel K, Spetlikova E, Sikurova L, Lukes P (2013) Formation of ROS and RNS in water electro-sprayed through transient spark discharge in air and their bactericidal effects. Plasma Process Polym 10(7):649–659
Naïtali M, Kamgang-Youbi G, Herry J-M, Bellon-Fontaine M-N, Brisset J-L (2010) Combined effects of long-living chemical species during microbial inactivation using atmospheric plasma-treated water. Appl Environ Microbiol 76(22):7662–7664
Wende K, Williams P, Dalluge J, Gaens WV, Aboubakr H, Bischof J, von Woedtke T, Goyal SM, Weltmann KD, Bogaerts A, Masur K, Bruggeman PJ (2015) Identification of the biologically active liquid chemistry induced by a nonthermal atmospheric pressure plasma jet. Biointerphases 10(2):029518
Traylor MJ, Pavlovich MJ, Karim S, Hait P, Sakiyama Y, Clark DS, Graves DB (2011) Long-term antibacterial efficacy of air plasma-activated water. J Phys D Appl Phys 44(47):472001
Oehmigen K, Winter J, Hähnel M, Wilke C, Brandenburg R, Weltmann K-D, von Woedtke T (2011) Estimation of possible mechanisms of escherichia coli inactivation by plasma treated sodium chloride solution. Plasma Process Polym 8(10):904–913
Naïtali M, Herry J-M, Hnatiuc E, Kamgang G, Brisset J-L (2012) Kinetics and bacterial inactivation induced by peroxynitrite in electric discharges in air. Plasma Chem Plasma Process 32(4):675–692
Pavlovich MJ, Chang H-W, Sakiyama Y, Clark DS, Graves DB (2013) Ozone correlates with antibacterial effects from indirect air dielectric barrier discharge treatment of water. J Phys D Appl Phys 46(14):1–10
Kanazawa S, Kawano H, Watanabe S, Furuki T, Akamine S, Ichiki R, Ohkubo T, Kocik M, Mizeraczyk J (2011) Observation of OH radicals produced by pulsed discharges on the surface of a liquid. Plasma Sour Sci Technol 20(3):034010
Bruggeman P, Schram DC (2010) On OH production in water containing atmospheric pressure plasmas. Plasma Sour Sci Technol 19(4):045025
Tian W, Kushner MJ (2014) Atmospheric pressure dielectric barrier discharges interacting with liquid covered tissue. J Phys D Appl Phys 47(16):165201
von Gunten U, Ramseier M (2010) Critical review of literature for rate constants for reaction of chemical oxidants with inorganic and organic pollutants. Dubendorf, Switzerland
Buxton GV, Greenstock CL, Helman WP, Ross AB (1988) Critical-review of rate constants for reactions of hydrated electrons, hydrogen-atoms and hydroxyl radicals (.OH/.O–) in aqueous-solution. J Phys Chem Ref Data 17(2):513–886
Halliwell B, Gutteridge JMC (2007) Free radicals in biology and medicine, 4th edn. Oxford University Press, Oxford
Lim CH, Dedon PC, Deen WM (2008) Kinetic analysis of intracellular concentrations of reactive nitrogen species. Chem Res Toxicol 21(11):2134–2147
NDRL/NIST Solution Kinetics Database (2016) NIST standard reference database 40, 2002. http://kinetics.nist.gov/solution/. Accessed 19 Jan 2016
Winter J, Tresp H, Hammer MU, Iseni S, Kupsch S, Schmidt-Bleker A, Wende K, Dünnbier M, Masur K, Weltmann K-D, Reuter S (2014) Tracking plasma generated H2O2 from gas into liquid phase and revealing its dominant impact on human skin cells. J Phys D Appl Phys 47:285401
Locke BR, Shih K-Y (2011) Review of the methods to form hydrogen peroxide in electrical discharge plasma with liquid water. Plasma Sour Sci Technol 20(3):034006
Wandell RJ, Locke BR (2014) Hydrogen peroxide generation in low power pulsed water spray plasma reactors. Ind Eng Chem Res 53:609–618
Goldstein S, Lind J, Merényi G (2005) Chemistry of peroxynitrites as compared to peroxynitrates. Chem Rev 105(6):2457–2470
Tresp H, Hammer MU, Winter J, Weltmann K-D, Reuter S (2013) Quantitative detection of plasma-generated radicals in liquids by electron paramagnetic resonance spectroscopy. J Phys D Appl Phys 46(43):435401
Uchiyama H, Zhao Q-L, Hassan MA, Andocs G, Nojima N, Takeda K, Ishikawa K, Hori M, Kondo T (2015) EPR-spin trapping and flow cytometric studies of free radicals generated using cold atmospheric argon plasma and X-ray irradiation in aqueous solutions and intracellular milieu. PLoS ONE 10(8):e0136956
Bader H, Hoigne J (1981) Determination of ozone in water by the indigo method. Water Res 15:449–456
Galindo C, Jacques P, Kalt A (2001) Photochemical and photocatalytic degradation of an indigoid dye: a case study of acid blue 74 (AB74). J Photochem Photobiol A Chem 141(1):47–56
Sychev AY, Isak VG, Pfannmeller U (1979) Determination of rate constants of hydroxyl radicals with organic and inorganic substances under conditions for the catalytic decomposition of hydrogen peroxide. Russ J Phys Chem 53:1595–1598
Kettle AJ, Clark BM, Winterbourn CC (2004) Superoxide converts indigo carmine to isatin sulfonic acid: implications for the hypothesis that neutrophils produce ozone. J Biol Chem 279(18):18521–18525
Sugiarto AT, Ito S, Ohshima T, Sato M, Skalny JD (2003) Oxidative decoloration of dyes by pulsed discharge plasma in water. J Electrostat 58:135–145
Magureanu M, Bradu C, Piroi D, Mandache NB, Parvulescu V (2013) Pulsed corona discharge for degradation of methylene blue in water. Plasma Chem Plasma Process 33(1):51–64
Grabowski LR, Van Veldhuizen EM, Pemen AJM, Rutgers WR (2007) Breakdown of methylene blue and methyl orange by pulsed corona discharge. Plasma Sour Sci Technol 16(2):226–232
Brisset J-L, Benstaali B, Moussa D, Fanmoe J, Njoyim-Tamungang E (2011) Acidity control of plasma-chemical oxidation: applications to dye removal, urban waste abatement and microbial inactivation. Plasma Sour Sci Technol 20(3):034021
Vautier M (2001) Photocatalytic degradation of dyes in water: case study of indigo and of indigo carmine. J Catal 201(1):46–59
Janda M, Martišovitš V, Machala Z (2011) Transient spark: a dc-driven repetitively pulsed discharge and its control by electric circuit parameters. Plasma Sour Sci Technol 20(3):035015
Zhao L, Adamiak K (2005) EHD flow in air produced by electric corona discharge in pin–plate configuration. J Electrostat 63:337–350
Machala Z, Jedlovský I, Martǐsovitš V (2008) DC discharges in atmospheric air and their transitions. IEEE Trans Plasma Sci 36(4):918–919
Janda M, Machala Z, Niklová A, Martišovitš V (2012) The streamer-to-spark transition in a transient spark: a dc-driven nanosecond-pulsed discharge in atmospheric air. Plasma Sour Sci Technol 21(4):045006
Janda M, Machala Z (2011) Imaging of transient spark in atmospheric air by fast iCCD camera. IEEE Trans Plasma Sci 39(11):2246–2247
Machala Z, Janda M, Hensel K, Jedlovský I, Leštinská L, Foltin V, Martišovitš V, Morvová M (2007) Emission spectroscopy of atmospheric pressure plasmas for bio-medical and environmental applications. J Mol Spectrosc 243(2):194–201
Eisenberg G (1943) Colorimetric determination of hydrogen peroxide. Ind Eng Chem Anal Ed 15(5):327–328
Satterfield CN, Bonnell AH (1955) Interferences in titanium sulfate method for hydrogen peroxide. Anal Chem 27(7):1174–1175
Hoigne J, Bader H (1983) Rate constants of reactions of ozone with organic and inorganic compounds in water—II. Water Res 17:185–194
Gordon G, Bubnis B (2001) Residual ozone measurement: indigo sensitivity coefficient adjustment. Ozone Sci Eng J Int Ozone Assoc 24:17–28
Babaeva NY, Tian W, Kushner MJ (2014) The interaction between plasma filaments in dielectric barrier discharges and liquid covered wounds: electric fields delivered to model platelets and cells. J Phys D Appl Phys 47(23):235201
Rumbach P, Bartels DM, Sankaran RM, Go DB (2015) The solvation of electrons by an atmospheric-pressure plasma. Nat Commun 6:7248
Ono R, Oda T (2002) Dynamics and density estimation of hydroxyl radicals in a pulsed corona discharge. J Phys D Appl Phys 35(17):2133–2138
Pacher P, Beckman JS, Liaudet L (2007) Nitric oxide and peroxynitrite in health and disease. Am Physiol Soc Physiol Rev 87:315–424
Roots R, Okada S (2014) Estimation of life times and diffusion distances of radicals involved in X-ray-induced DNA strand breaks or killing of mammalian cells. Radiat Res 64(2):306–320
Takeuchi K, Ibusuki T (1989) Quantitative determination of aqueous-phase ozone by chemiluminuescense using indigo-5,5′-disulfonate. Anal Chem 61(6):619–623
Ono R, Oda T (2003) Dynamics of ozone and OH radicals generated by pulsed corona discharge in humid-air flow reactor measured by laser spectroscopy. J Appl Phys 93(10):5876
Wu H, Sun P, Feng H, Zhou H, Wang R, Liang Y, Lu J, Zhu W, Zhang J, Fang J (2012) Reactive oxygen species in a non-thermal plasma microjet and water system: generation, conversion, and contributions to bacteria inactivation-an analysis by electron spin resonance spectroscopy. Plasma Process Polym 9(4):417–424
Tabrizchi M, Khayamian T, Taj N (2000) Design and optimization of a corona discharge ionization source for ion mobility spectrometry. Rev Sci Instrum 71(2000):2321
Bruggeman P, Iza F, Lauwers D, Gonzalvo YA (2009) Mass spectrometry study of positive and negative ions in a capacitively coupled atmospheric pressure RF excited glow discharge in He–water mixtures. J Phys D Appl Phys 43(1):012003
Skalny JD, Orszagh J, Matejcik S, Mason NJ, Rees JA, Aranda-Gonzalvo Y, Whitmore TD (2008) A mass spectrometric study of ions extracted from point to plane DC corona discharge fed by carbon dioxide at atmospheric pressure. Int J Mass Spectrom 277(1–3):210–214
Joshi SG, Cooper M, Yost A, Paff M, Ercan UK, Fridman G, Friedman G, Fridman A, Brooks AD (2011) Nonthermal dielectric-barrier discharge plasma-induced inactivation involves oxidative DNA damage and membrane lipid peroxidation in Escherichia coli. Antimicrob Agents Chemother 55(3):1053–1062
Aboubakr HA, Gangal U, Youssef MM, Goyal SM, Bruggeman PJ (2016) Inactivation of virus in solution by cold atmospheric pressure plasma: identification of chemical inactivation pathways. J Phys D Appl Phys 49(20):204001
De Melo JSS, Rondão R, Burrows HD, Melo MJ, Navaratnam S, Edge R, Voss G (2006) Spectral and photophysical studies of substituted indigo derivatives in their keto forms. ChemPhysChem 7(11):2303–2311
Gandra N, Frank AT, Le Gendre O, Sawwan N, Aebisher D, Liebman JF, Houk KN, Greer A, Gao R (2006) Possible singlet oxygen generation from the photolysis of indigo dyes in methanol, DMSO, water, and ionic liquid, 1-butyl-3-methylimidazolium tetrafluoroborate. Tetrahedron 62(46):10771–10776
Nakagawa Y, Ono R, Oda T (2011) Density and temperature measurement of OH radicals in atmospheric-pressure pulsed corona discharge in humid air. J Appl Phys 110(7):1–7
Ono R, Oda T (2008) Measurement of gas temperature and OH density in the afterglow of pulsed positive corona discharge. J Phys D Appl Phys 41(3):035204
Witzke M, Rumbach P, Go DB, Sankaran RM (2012) Evidence for the electrolysis of water by atmospheric-pressure plasmas formed at the surface of aqueous solutions. J Phys D Appl Phys 45(44):442001
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Anderson, C.E., Cha, N.R., Lindsay, A.D. et al. The Role of Interfacial Reactions in Determining Plasma–Liquid Chemistry. Plasma Chem Plasma Process 36, 1393–1415 (2016). https://doi.org/10.1007/s11090-016-9742-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11090-016-9742-1