Abstract
Several modern power production systems utilize supercritical CO2 (sCO2), which can contain O2 and H2O as impurities. These impurities may degrade the compatibility of structural alloys through accelerated oxidation. However, it remains unclear which of these impurities plays a bigger role in high-temperature reactions taking place in sCO2. In this study, various model and commercial Fe‐ and Ni‐based alloys were exposed in 300 bar sCO2 at 750 °C to low levels (50 ppm) of O2 and H2O for 1,000 h. 18O-enriched water was used to enable the identification of the oxygen source in the post-exposure characterization of the samples. However, oxygen from the water did not accumulate in the scale, which consisted of Cr2O3 in the cases where a protective oxide formed. A 2wt.% Ti addition to a Ni-22%Cr model alloy resulted in the formation of thicker oxides in sCO2, while a 1wt.% Al addition reduced the scale thickness. A synergistic effect of both Al and Ti additions resulted in an even thicker oxide than what was formed solely by Ti, similar to observations for Ni-based alloy 282.
Similar content being viewed by others
Introduction
Increasing electricity demand and reductions in CO2 emissions are key considerations when designing new power plants with higher production efficiencies. The so-called Brayton cycle is a thermodynamic cycle used as the basis of constant-pressure heat engines in modern jet engines and gas turbines. The Brayton cycle can also be used with supercritical carbon dioxide (sCO2) as a working fluid in modern power production systems regarding nuclear energy, concentrated solar power (CSP), and emission-free fossil energy [1,2,3]. When compared to the steam-based Rankine cycle, the thermophysical properties of sCO2 allow higher cycle efficiencies, smaller environmental impact, and lower costs due to simpler power block design [4,5,6]. The use of sCO2 could enable efficiencies over 50% with a sCO2 Brayton cycle operating above 700 °C and 200–300 bar (20–30 MPa) [7].
So far, most of the studies addressing high-temperature oxidation of materials in sCO2 have been performed in research-grade (RG) sCO2 (purity 99.999%) [i.e., 8–13]. Although various materials, especially higher-alloyed ones, perform satisfactorily in RG sCO2, it has been reported to initiate breakaway corrosion of Cr-rich stainless steel through the growth of an Fe-rich oxide and rapid precipitation of internal Cr carbides [14] due to C ingress into the alloy [15]. Since Cr is bound to carbides, the bulk alloy becomes Cr-depleted and the repassivation of the alloy surface through Cr diffusion is inhibited. Increasing the Cr content of the alloy results in a better resistance toward carburization after the formation of a thin and continuous chromia scale [12]. However, it was reported that an amorphous carbon layer together with Cr-rich carbides (M23C6) was found below the oxide, indicating that the chromia scale might be, to some extent, permeable to carbon. The low oxygen partial pressure p(O2) at the scale/alloy interface enables the following reactions to occur:
The reactions above result in a carbon activity high enough to initiate internal carburization [16, 17]. Alumina scales have been found to withstand carburization better than chromia scales [18, 19]. However, the alumina scale is impermeable to carbon only when it is continuous and consists of α-alumina [20]. Interestingly, Ni-based alloys appear to withstand carburization better than Fe-based alloys, most likely due to the reduced carbon solubility and Cr carbide stability in Ni-based alloys [14, 21]. Unfortunately, Ni-based materials are considered to be too expensive for many industrial applications.
In addition to studies in research-grade sCO2, the impact of impurities such as O2 and H2O on high-temperature oxidation kinetics in sCO2 has been addressed, although the amount of published information is very limited. Most of the studies were carried out at ambient pressure. It has been suggested that system pressure has a negligible effect on the oxidation rate of both Fe- and Ni-based alloys in sCO2 [13, 22]. However, this may not be the case with impurity effects [23,24,25]. Thus, impurity studies at high pressures are of interest because, for example, H2O and O2 might react with the protective chromia scale and form volatile Cr oxy-hydroxides such as CrO2(OH)2, especially in open cycles with high H2O levels [23, 26]. Chromium volatilization depletes Cr in the scale and the alloy beneath it, accelerating the degradation of high-alloyed materials significantly. The effects of impurities on high-temperature oxidation in sCO2 and specifically the reaction mechanisms are still not well defined. The effect of O2 (0.15 vol.%) or H2O (10 vol.%) additions to CO2 (0.1 MPa) at 700 °C resulted in very similar mass changes than what was observed in pure CO2 [27]. The thicknesses of the scales formed at 700 °C in air, CO2, or in CO2 + H2O were also very close to one another. Interestingly, the addition of either H2O or O2 to CO2 has also been reported to result in faster oxidation rates for both Fe- and Ni-based alloys [23, 24]. The detrimental effect of oxygen was suggested to originate from accelerated Cr diffusion and void formation [24]. The presence of H2O may result in an increased number of grain boundaries, which function as diffusion routes for carbon, leading to carburization of the alloy beneath the scale [28]. The different results above indicate the necessity of further studies addressing the role of impurities on oxidation reactions and material degradation in sCO2.
The goal of the current study was to better understand the impurity-induced corrosion taking place in CO2 (300 bar) at 750 °C with low levels of impurities that might be found in closed cycles. For this, known amounts of O2 and H2O were added to the sCO2 experiments, in which corrosion of model and commercial FeCr- and NiCr-alloys was studied. The effects of Al and Ti additions were investigated because of their importance in strengthening Ni-based alloys, like alloy 282 [29]. To differentiate the role of the two impurities, 18O isotope-enriched water was utilized. Thus, the additional focus lies on the applicability of tracer studies in high-temperature CO2 environments.
Experimental
Two commercial and five model alloys (compositions in Table 1) were cut into rectangular pieces (12 mm × 20 mm) with a thickness of ~ 1 mm and polished to a 600 grit finish. For each alloy, two samples were weighed before the experiments using a Mettler Toledo XP205 balance with an accuracy of ~ ± 0.04 mg or 0.01 mg/cm2. The experiments were carried out in a vertical autoclave in research-grade (purity 99.999%) CO2 under 300 bar at 750 °C. The autoclave (~ 266 mm × 83 mm inner diameter) was operated inside a three-zone furnace with an alloy 282 sample rack that sat on the bottom of the autoclave. The liquid CO2 flow rate was around 0.1 kgh−1. In addition, known amounts (50 ppm) of impurities (O2 and H2O) were introduced into the autoclave with pumps specially designed for high-pressure environments. Molecular oxygen (purity 99.999%) contained 99.8 vol% of the 16O isotope, whereas the water was 18O isotope-enriched, containing almost exclusively the 18O isotope (18O content > 98%, GMP quality, Rotem Industries Ltd.). The specimens were heated to temperature ~ 3 °C min−1 in sCO2, held at temperature ± 2 °C, and then cooled in sCO2 to room temperature by lowering the furnace. One sample of each alloy was removed after 500 h and the other one after 1,000 h, after which the samples were weighed.
Time of flight secondary-ion mass spectrometry (ToF–SIMS) was used to identify the two oxygen isotopes, for elemental depth profiling of the oxide scales, and for chemical characterization of the bulk alloys. The measurements were performed using a TOF.SIMS.5 NSC instrument (IONTOF GmbH) with a Bi3+ liquid metal ion gun (energy of 30 keV, current ~ 30 nA, and spot size ~ 5 μm) as the primary source for chemical analysis on the surface. In addition, a Cs+ ion gun (energy of 2 keV, current ~ 100 nA, and spot size ~ 20 μm) was used as a sputter source for depth profiling. Measurements were performed in non-interlaced mode, with each chemical imaging over a 100 × 100 μm area followed by 25 s of the sputtering over a 200 × 200 μm area. Mass analysis of secondary ions was realized using a time-of-flight mass analyzer operating in positive ion detection mode and providing mass resolution m/Δm = 5,000–10,000. Information from the analyzed area was averaged over X–Y coordinates and further presented as depth profiles for chemical elements of interest.
The oxide phases in the scales were identified with X-ray diffraction (XRD) by a Panalytical X’pert diffractometer from nominally 5 to 92° 2θ using CuKα radiation (λ = 1.540598 Å). All scans used ¼° fixed slits, ½° anti-scatter slit, 0.04 soller slits coupled with a 10-mm mask (beam length). For the phase identification procedure, a search match was conducted using the Jade software [30] and the ICDD database [31]
After the abovementioned characterization, the samples were copper plated, sectioned, and cast in epoxy to be studied with a light optical microscope (LOM) and scanning electron microscope (SEM). The SEM (Hitachi S4800) with an energy-dispersive X-ray analysis system (EDAX Octane Elect EDS System) was used to study the chemical composition and structure of the formed oxides from cross-sectional samples. The microscope was operated at an accelerating voltage of 20 kV and with the backscatter electron mode for the images and EDX analyses.
Results
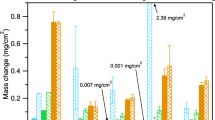
The weight changes after 500 and 1,000 h are presented in Fig. 1. For comparison, the mass gains from exposure in industrial grade (IG) sCO2 also are shown for several alloys. In Fig. 2, the thickness of each formed scale was calculated as an average of twenty thickness measurements and the calculated parabolic rate constants in Table 2. Only the external oxide has been taken into account and any internal degradation has been excluded from the thickness measurements. Since similar alloys have been reported to follow parabolic oxidation kinetics [32, 33], Eq. 3 has been used to calculate parabolic rate constants presented in Table 2.
The cross-sectional images in Fig. 3 show a general trend of internal attack mainly along the grain boundaries in all the alloys containing Ti. The internal attack has formed cavities along the grain boundaries [35], most likely as a result of the outward migration of Ti. Additionally, minor signs of internal degradation were also observed in the Ni22Cr + 1Al model alloy.
The polished cross sections were also examined by SEM, which showed internal degradation of Ti-containing alloys and the Ni22Cr + 1Al model alloy even clearer (Fig. 4). Based on the SEM–EDX elemental maps, chromia is the main protective oxide with all presented alloys (examples in Figs. 5 and 6). In addition to Cr, Ti was identified in the surface scales of all Ti-containing alloys (282, Ni22Cr + 2Ti, and Ni22Cr + 1Al,2Ti). The presence of Ti in the surface oxides was also verified with XRD (Table 3). Based on the identified phases, Ti was presented as pure rutile (TiO2) and in titanium-doped chromia with the chromia structure ((Cr,Ti)2O3) [36]. Unfortunately, the exact oxide compositions cannot be determined from the XRD patterns alone. The alloying elements in the bulk material may be embedded in the oxide structure, which leads to peak shifts compared to pure oxides. This originates from Fe or Ti atoms substituting Cr in the lattice and creating a slight distortion in the peak positions, which results in the identification of additional phases. To avoid intricacy, notations (Cr,Fe)2O3 and (Cr,Ti)2O3 are presented in Table 3 instead of exact phase stoichiometries. Based on the SEM images, Al did not form a continuous alumina scale below the chromia scale, thus failing to protect the alloy from further attack. The lack of a protective barrier explains the signs of incipient internal attack shown in Fig. 3.
Based on the ToF–SIMS depth profiles, the exposure time did not affect the chemical composition of the scales, only their thicknesses. The chemical compositions obtained with sputtering agreed well with the other analyses. The only additional finding was the Mn-rich surface layer on the HR3C alloy (Figs. 7 and 8). The layer was so thin that it was not detected by XRD or SEM–EDS, but it consisted most likely of a (Mn,Cr)3O4 spinel, typically found on Mn-containing alloys exposed to sCO2 [37].
Discussion
Mass Change
The largest mass gain was observed with the Fe15Cr model alloy, peaking at around 3.1 mgcm−2 after 500 h. Based on visual observation, heavy spallation occurred on the 1,000-h sample, explaining the notably lower mass gain. Both the high mass gain and spallation indicate that under the studied conditions, 15wt.% of Cr in the Fe-based model alloy is not enough to provide corrosion resistance, despite the Cr-rich oxide identified by XRD ((Cr,Fe)2O3). Even so, this mixed oxide scale was not protective and could not slow the rate of oxidation. Information addressing the scale composition after 1000 h was lost due to spallation and no further characterization was conducted on the spalled specimen. However, it has been reported with commercial Fe-based alloys with similar Cr contents that a thick Fe3O4 scale formed in sCO2 at 650 °C [37]. The spallation of the scale can be attributed to growth stresses within the scale due to volume changes during oxidation and to the discrepancy between the thermal expansion coefficients of the alloy and the scale. The external chromia formation depletes the oxide scale/alloy interface in Cr, in addition to which chromium carbide (Cr23C6) precipitation is reported to decrease the chromium level further [14]. After spallation, the regrowth of the chromia scale is hindered due to the Cr-depleted region, and iron oxide nodules may form instead.
Unfortunately, the amount of the stable 18O isotope found in all of the exposed samples was within the natural abundance of the isotope (0.2%). It remains unclear, whether H218O reacted with the alloys at all. However, it did not accumulate within the scale during the 1,000 h exposure. This suggests that water might not have a major role in accelerated oxidation as an impurity in sCO2. However, more research needs to be carried out to verify the relative effects of O2 and H2O. For example, repeating the current experiment with only 50 ppm O2 or only 50 ppm H2O additions. A summary of the various environments investigated is shown in Fig. 9. The emphasis has been on higher O2 and H2O levels in support of the planned Allam cycle [3] commercialization. For HR3C, the results suggested that 1% O2 alone had a more detrimental effect than adding 0.25% H2O [38]. The reactivity of O2 was found higher also when compared to CO2 when a mild steel was exposed to gas mixtures with different ratios of O2 and CO2 [39]. The initial oxidation of the steel was attributed almost exclusively to O2 due to its faster dissociation rate. Once all O2 has reacted and if no additional O2 is available, the oxidation of the steel slows down and originates from the presence of CO2. So, O2 dominates the pO2 when present, and under the studied conditions, the pO2 was 0.015 bar. The pO2 for pure sCO2 has been reported earlier in [22] and it was concluded to be almost as high as in steam, so all of the typical Fe and Ni oxides are stable, almost up to pO2 of 10–5 bar.
The Effect of Al Addition in Ni–Cr Model Alloys
For the Ni22Cr model alloys, the oxide scale consisted mainly of chromia, and the oxide thickness increased in series Ni22Cr + 1Al < Ni22Cr < Ni22Cr + 2Ti < Ni22Cr + 1Al,2Ti. This is in good accordance with the general knowledge that when only aluminum is alloyed, it slows down the oxidation rate compared to a similar Al-free alloy [40, 41]. Usually, the positive effect of Al is attributed to the formation of a dense and protective α-alumina scale, mainly through inward-diffusion of oxygen along short-circuit paths such as grain boundaries [42]. The formation of α-alumina scale is dominant over transient aluminas at temperatures above 800 °C [43, 44]. In sCO2, a continuous alumina scale can form already at 650 °C, but it requires higher Al contents (> 3wt.%) [45, 46]. Since neither XRD nor EDS could detect a subscale alumina layer, it appears that 1% Al was not sufficient to form an Al-rich layer at 750 °C in agreement with the Ni–Cr-Al map of Giggins and Pettit [43].
In the present study, the oxygen potential at the chromia-alloy interface was higher than what is required to oxidize Al, and therefore, alumina precipitates formed beneath the chromia scale as can be seen in Figs. 5 and 6. Although 2% of Al in the alloy has been reported to be sufficient for the formation of a continuous alumina scale underneath the external chromia scale in air at 800 °C [47], 1% Al in the present study was not sufficient to form a continuous internal alumina scale at 750 °C in sCO2. The formation of a discontinuous alumina scale has been reported previously in air [43] and in sCO2 at lower temperatures [48]. Interestingly, the presence of Al precipitates or a discontinuous subscale layer in the internal oxidation zone (IOZ) was sufficient to reduce the scale thickness. In NiCrAl alloys, the oxidation of both Cr and Al at the scale-alloy surface results in an increased oxide volume fraction and promoted formation of a Cr-rich oxide at the scale-IOZ interface [48]. This uniform chromia scale in Fig. 6 reduces the oxygen activity at the scale-IOZ interface, resulting in slower oxidation and outward diffusion of metal ions. This may explain the lower mass gain and thinner oxide of the Ni22Cr + 1Al model alloy compared to the undoped Ni22Cr model alloy. Since no Ni-rich scales were identified by XRD in the present study and both mass gain and oxide thickness of the Ni22Cr + 1Al model alloy were smaller than those of the Ni22Cr model alloy, Al appears to have a beneficial effect on the oxidation behavior although the added 1% could not entirely prevent internal attack. So, as long as the Cr content of the alloy is high enough, a 1% Al addition provided further protection for the alloy.
The Effect of Cr Content in Commercial Alloys
Based on the mass gains and oxide thicknesses of HR3C and 282, it appears that in sCO2 at 750 °C, the Cr content of the alloy has a bigger beneficial impact on corrosion resistance than a small Al addition. The effect of higher Cr content can also be observed when comparing the oxidation behavior of Fe-15Cr to that of HR3C. For the tracer study, it was hoped that the binary Fe-15Cr alloy would facilitate analysis. Unfortunately, 15Cr was not sufficient to prevent rapid Fe-rich oxide formation. Regarding the two commercial alloys, HR3C and 282, the higher mass gain for 282 is attributed to the oxidation of Al and Ti, which are not present in HR3C (Table 1). For HR3C, there was also Mn present in the scale as an Mn-rich (Mn,Cr)3O4 spinel, which may be beneficial in terms of oxidation resistance [34, 49]. At low O partial pressure, which is expected in sCO2, Mn additions reduce the oxidation rate remarkably by slowing metal and/or oxygen diffusion as well as blocking carbon penetration [50, 51]. This is explained by the pO2-dependence of Mn solubility in Cr2O3 and in the resulting Cr/Mn-spinel: at low pO2 values, a stoichiometric spinel (MnCr2O4) forms, whereas at higher pO2 values, Mn starts to occupy chromium sites in the oxide, forming a continuous solid solution of (MnxCr1-x)3O4 [50, 52]. When compared to mass gains in IG sCO2 after 1,000 h (Fig. 1), the differences were negligible for alloy 282 and small for alloys HR3C and NiCr22 in the current study.
The Effect of Ti Addition
Under these test conditions, the presence of Ti resulted in faster scale growth, which was further pronounced when both Al and Ti were present. All three Ti-containing alloys in the present study had high enough Ti contents to form an external TiO2 layer beneath the chromia scale [48], but since TiO2 is soluble in Cr2O3 [53], no such layer formed, and the Ti diffusion through the chromia scale formed an outermost TiO2 layer instead. The fact that TiO2 was detected in the scale supports the idea of Ti-enhanced sintering of the scale and the oxidation of the alloy due to alteration of the defect structure of the oxide scale [54].
The formation of cavities in the exposure to sCO2 differs somewhat from the type of internal attack in air, where the grain boundaries are usually oxidized due to the very high oxygen affinity of Ti [55]. Signs of internal attack were visible in the model alloys containing Al and/or Ti. Both Al and Ti form more stable oxides than Cr2O3 and thus can oxidize beneath the scale at O partial pressures below the Cr/Cr2O3 equilibrium established at the metal-scale interface. Without Al and Ti, the oxides formed on the samples HR3C, Fe15Cr, and Ni22Cr were dense, well-adhered, and without significant internal oxidation as can be seen in Fig. 4. Titanium is known to be an oxygen active element [56], but its effect depends on prevailing conditions and the composition of the base alloy. At temperatures above 900 °C, Ti additions have been reported to enhance oxidation resistance of Co–Ni-based superalloys [57] and FeAl intermetallics [58], whereas Ni-based model alloys typically show faster scale growth with Ti additions at 800 °C [59,60,61]. The higher mass gain of the Ti-containing model alloys in the current study supports a Ti4+ doping mechanism in Cr2O3. The Ti ions detected in the chromia scale by XRD are occupying the regular chromium sites, because Ti4+ and Cr3+ have almost the same ionic radius. The Ti occupation results in a positive effective charge (Ti4+ replacing Cr3+), which needs to be compensated either by the formation of chromium vacancies or electrons [62, 63]. In a previous study, where the oxidation mechanism of Ti-containing FeCr alloys was studied, Ti was found within the chromia scale at the grain boundaries with high enough concentrations to generate bulk vacancies via the Wagner-Hauffe doping [64, 65]. The formation of cation vacancies contributes to the ionic mass transport, which results in accelerated outward cation diffusion along chromia grain boundaries and eventually, in increased oxidation rate as can be seen in Fig. 2. Another possible effect of the presence of Ti ions in the chromia scale is the grain refinement of the chromia grains, which also increases the oxide growth [66, 67]. The EDS analyses in Fig. 6 indicated that the thick scales on the Ti-containing alloys consisted mainly of chromia, which can be seen as a positive effect, despite the higher mass gain. This is based on the observation that faster chromia formation suppresses the detrimental formation of a NiO scale [48]. Furthermore, Ti–rich precipitates in the IOZ have been reported to reduce the growth of IOZ remarkably [48]. However, this could not be verified in the present study. Instead, the rapid Ti and Cr diffusion resulted in a notable internal attack. Although outside the scope of this paper, it should be mentioned that the formation of a thick surface oxide together with internal attack of Ti (and Al) may deteriorate the alloy’s capability to resist mechanical stress by dissolving the γ' strengthening phase. This has been observed in cyclic tests, where both the surface oxide and internal voids led to crack initiation, subsequent propagation into the substrate, and ultimately, material failure [68]. This shows that on a general level, Ti additions affect negatively the defect structure and oxidation kinetics of the scale, depending on i) temperature, ii) Ti concentration, and iii) O partial pressure [53, 69]. Nevertheless, Ti is important for the formation of the γ' strengthening phase and is necessary to achieve the exceptional creep strength in an alloy like 282 [29].
The mass gains and oxide thicknesses correlate quite well with one another and the calculated rate constants are in the same order of magnitude as those of commercial alloys studied under similar conditions [32, 38, 70]. Interestingly, the oxidation rate constant for 282 alloy (kp = 2.9 × 10–8 mg2cm−4 s−1) in sCO2 was close to that of a Ni-based alloy with similar Cr, Ti, and Al concentrations (kp = 1.7 × 10–8 mg2cm−4 s−1), when studied in air at 750 °C for 2000 h [63]. It might be that oxygen availability in sCO2 was high enough to oxidize highly reactive Ti and Al in alloy 282 at a similar rate than in air, which is also pointed out in Fig. 9.
The Combined Effect of Ti and Al Additions
Interestingly, the addition of Al to the model alloy with Ti actually increased the scale thickness and the depth of internal attack. Less Ti in the form of Ti0.24Cr1.76O3 was identified in the oxide of the Ni22Cr + 2Ti model alloy, when compared with the Ni22Cr + 1Al,2Ti model alloy, where both Ti0.24Cr1.76O3 and TiO2 formed. This suggests that the presence of Al in the alloy enhanced the migration of Ti toward the surface, where it formed a mixed oxide, similarly to alloy 282. The impact of simultaneous Ti and Al additions is not frequently addressed in the literature, and therefore, the oxidation mechanism originating from the possible interaction between Al and Ti and the reason for this synergistic effect is not resolved. When a similar Ni22Cr + 2Al,1Ti model alloy was exposed in air at 800–950 °C, a higher mass gain was recorded than for a Ni22Cr + 2Al model alloy [47]. It was hypothesized that the alumina underlayer may hinder Cr diffusion and the growth of the chromia scale but is unable to prevent the transport of Ti through the oxide scale. This results in the incorporation of Ti in the scale (Fig. 6) and subsequent oxidation leading to the higher mass gain. When the high-temperature oxidation of high-entropy alloys (HEAs) was studied, Ti addition to an Al-containing alloy formed oxide phases with poor oxidation resistance [71]. However, the chemical compositions of the studied alloys differ so much from the alloys in the present study that no direct comparison can be made. Since alumina is more stable than TiO2, one possibility is that the addition of Al results in more Al internal oxidation and a lower Al/Al2O3 oxygen partial pressure with less Ti internal oxidation. Internal oxidation of Ti still occurs but to a lesser extent. Rather than forming Ti internal oxides, more Ti is available to diffuse outward, increasing the doping of the scale (i.e., faster growth) and forming more Ti–rich precipitates (TiO2 and Ti-mixed oxides, Table 3) at the gas/scale interface.
The Effect of Mn and Si Additions
The original goal was to compare sCO2 impurity effects on Fe- and Ni-based commercial and model alloys. As already noted, the 50 ppm level impurities produced little effect on either commercial alloy (Fig. 1). The thinner scale formed on HR3C compared to 282 (Figs. 3 and 4) can be attributed to the Al and Ti in the latter. However, HR3C also contains Mn and Si that are not typically added to Ni-based alloys. The detected outer (Mn,Cr)3O4-type spinel layer was similar to what has previously been observed in steam oxidation of HR3C at 700 °C [72]. Silicon formed a continuous layer below the chromia scale in HR3C, which might partly explain the low mass gain of this alloy. When Ni-based model alloys were exposed to CO2 at 700 °C, Si additions improved the oxidation resistance of the alloys significantly [48]. The beneficial effect of Si originates from the formation of a SiO2 layer at the scale–alloy interface, where it may inhibit transport in the chromia scale and thus reduce Cr depletion in the underlying alloy, which can be seen in the Cr maps in Fig. 5. The same effect applies to Fe-based model alloys and the oxidation rate was suppressed as a function of Si content in the range of 0.1–0.5wt.% [73]. HR3C contains 0.4wt.% of Si, which appears to be enough to form an Si-rich oxide sublayer. However, this sublayer is not always continuous at higher magnification [74]. When a Fe-20Cr-20Ni model alloy was doped with 0.2wt.% Si, the oxidation resistance in Ar-20CO2 at 818 °C enhanced through slower Cr depletion and chromia growth [75], which might have been the case with HR3C in the present study. Even if the silica layer would not be continuous, the presence of a discontinuous network of silica precipitates could slow down the oxidation rate. This takes place via the inhibition of the counter flow of Cr vacancies from the chromia scale into the bulk alloy [76]. The silica precipitates act as sinks, reducing the number of Cr atoms entering the external scale. In addition to the lower oxidation rate, the silica layer between the oxide scale and the alloy has been reported to improve scale adhesion [77] although a high Si level (> 0.5%) usually increases scale spallation. A silica layer also could suppress internal carburization by blocking the inward diffusion of carbon [78]. An alternative explanation is that Si affects the Cr diffusion rate in the alloy [79]. Also, since HR3C does not contain any Al or Ti, it benefits from not forming internal oxides, which partly explains the low mass gain.
Conclusions
The oxidation behavior of five model and two commercial alloys was studied in sCO2 at 750 °C for 1,000 h in the presence of 50 ppm of O2 and 50 ppm H2O as impurities. The used H2O was enriched with the stable 18-O isotope so that the oxygen atoms in water molecules could be separated from the 16-O atoms in molecular oxygen. Unfortunately, no 18O isotope could be detected after exposure, suggesting that H2O did not play a major role in the reaction when added at the 50 ppm level.
The key findings of the study can be summarized as:
-
Oxygen from the water did not accumulate in the forming oxide.
-
Chromium oxide was the main protective barrier against accelerated oxidation at 750 °C in sCO2.
-
The presence of Ti in the alloy resulted in the formation of thicker oxides on Ni-22Cr model alloys in sCO2.
-
When 1% Al was added to Ni-22Cr, a thinner Cr2O3 scale formed but this level of Al was not sufficient to form an alumina layer.
-
A synergistic effect of a combined Al and Ti addition resulted in an even thicker oxide than what was formed solely by Ti, perhaps reducing internal Ti oxidation and enhancing the diffusion of Ti into the surface oxide.
References
V. Dostal, P. Hejzlar, and M. J. Driscoll, Nuclear Technology 154, 265 (2006).
B. D. Iverson, T. M. Conboy, and J. J. Pasch, A. M. Kruizenga, Applied Energy 111, 957 (2013).
R. J. Allam, M. R. Palmer, G. W. Brown Jr and J. Fetvedt, D. Freed, H. Nomoto, M. Itoh, N. Okita, C. Jones Jr, Energy Procedia 37, 1135 (2013).
R. Moore and T. Conboy, Metal corrosion in a supercritical carbon dioxide – liquid sodium power cycle, Sandia Report SAND2012–0184, Sandia National Laboratories, Albuquerque, New Mexico, Livermore, California, (2012).
K. Lovegrove and J. Pye, Chapter 2 – Fundamental principles of concentrating solar power (CSP) systems, eds. K. Lovegrove, W. Stein, in Concentrating Solar Power Technology, (Woodhead Publishing, 2012), p. 16.
M. Mehos, C. Turchi, J. Vidal, and M. Wagner amd Z. Ma, Concentrating solar power Gen3 demonstrating roadmap, Technical Report NREL/TP-5500–67464, National Renewable Energy Laboratory, Golden, Colorado, (2017).
E. G. Feher, Energy Conversion 8, 85 (1968).
V. Firouzdor, K. Sridharan, G. Cao, M. Anderson, and T. R. Allen, Corrosion Science 69, 281 (2013).
G. Cao, V. Firouzdor, K. Sridharan, M. Anderson, T. R. Allen, Corrosion Science 60, 246 (2012).
B. Adam, L. Teeter, J. Mahaffey, M. Anderson, L. Árnadóttir, and J. D. Tucker, Oxidation of Metals 90, 2018 (453).
R. I. Olivares, D. J. Young, T. D. Nguyen, and P. Marvig, Oxidation of Metals 90, 2018 (1).
H. J. Lee, H. Kim, S. H. Kim, and C. Jang, Corrosion Science 99, 227 (2015)
R. P. Oleksak, J. H. Tylczak, C. S. Carney, and G. R. Holcomb, O. N. Doğan, JOM 70, 1527-1534 (2018).
R. I. Olivares, D. J. Young, P. Marvig, and W. Stein, Oxidation of Metals 84, 585 (2015).
H. E. McCoy, Corrosion 21, 84 (1965).
T. Gheno, D. Monceau, J. Zhang, and D. J. Young, Corrosion Science 53, 2011 (2767).
D. J. Young, P. Huczkowski, T. Olszewski, T. Hüttel, L. Singheiser, W. J. Quadakkers, Corrosion Science 88, 161 (2014).
B. Jönsson and C. Svedberg, Materials Science Forum 251–254(Pt. 2, High Temperature Corrosion and Protection of Materials 4, Pt. 2), 551 (1997).
I. E. McCarroll, A. La Fontaine, T. D. Nguyen, A. F. Smith, J. Zhang, D. J. Young, and J. M. Cairney, Corrosion Science 139, 2018 (267).
H. J. Lee, S. H. Kim, H. Kim, and C. Jang, Applied Surface Science 388(Part A), 483–490 (2016).
G. O. Subramanian, H. J. Lee, S. H. Kim, and C. Jang, Oxidation of Metals 89, 683 (2018)
B. A. Pint, R. G. Brese, and J. R. Keiser, Materials and Corrosion 68, 151 (2017).
B. A. Pint, J. Lehmusto, M. J. Lance, and J. R, Keiser, Materials and Corrosion 70, 1400 (2019)
J. Mahaffey, D. Adam, A. Brittan, M. Anderson, and K. Sridharan, Oxidation of Metals 86, 567 (2016)
J. Mahaffey, A. Schroeder, D. Adam, A. Brittan, M. Anderson, A. Couet and K. Sridharan, Metallurgical and Materials Transactions A 49, 3703 (2018).
D. J. Young and B. A. Pint, Oxidation of Metals 66, 2006 (137).
B. A. Pint and J. K. Thomson, Materials and Corrosion 65, 2014 (132).
T. D. Nguyen, A. La Fontaine, L. Yang, J. M. Cairney, J. Zhang, D. J. Young, Corrosion Science 132, 125 (2018).
L. M. Pike, Development of a fabricable gamma-prime (γ’) strengthened superalloy, in Superalloys, eds. Reed, R.C. et al. , TMS, Warrendale, PA, 2008, 191 (2008).
Jade (computer software), Materials Data Inc., Livermore, CA 94550, USA.2010.
ICDD, PDF-4+ 2018, International Centre for Diffraction Data, Newtown Square, PA.
J. Lehmusto, J. M. Kurley, M. J. Lance, and J. R. Keiser and B. A. Pint, Oxidation of Metals 94 , 95 (2020).
B. A. Pint, R. Pillai, M. J. Lance, and J. R. Keiser, Oxidation of Metals 94, 505 (2020)
B. A. Pint and K. A. Unocic, JOM 70, 1511 (2018).
R. P. Oleksak, J. H. Tylczak, G. R. Holcomb, and O. N. Dogan, Corrosion Science 157, 20 (2019).
T. D. Reynolds, M. P. Taylor, D. J. Child, and H. E. Evans, Materials at High Temperatures 35, 130 (2018).
H. Chen, S. H. Kim, C. Kim, J. Chen, C. Jang, Corrosion Science 156, 16 (2019).
B. A. Pint, K. A. Unocic, J. R. Keiser, Effect of impurities on supercritical CO2 compatibility, in Proc. 3rd European Conference on Supercritical CO2 (sCO2) Power Systems, 2019, 238–244.
F. Rouillard and D. J. Young, Corrosion Science 178, 109092 (2021).
H. Nagai and M. Okabayashi, Transactions of the Japan Institute of Metals 23, 334 (1982).
R. Elger and R. Pettersson, Oxidation of Metals 82, 469 (2014).
K. P. R. Reddy, J. L. Smialek, A. R. Cooper, Oxidation of Metals 17, 429 (1982)
C. S. Giggins and F. S. Pettit, Journal of The Electrochemical Society 118, 1782 (1971).
I. Goro and I. Seiichi, Nippon Kinzoku Gakkaishi 31, 1036 (1967).
H. Chen, S. H. Kim, C. Long, C. Kim, C. Jang, Progress in Natural Science: Materials International 28, 731 (2018).
V. Firouzdor, G. P. Cao, K. Sridharan, M. Anderson, and T. R. Allen, Materials and Corrosion 66, 137 (2015).
R. Pillai, M. Romedenne, J. A. Haynes, and B. A. Pint, Oxidation of Metals 95, 157 (2021).
T. D. Nguyen, J. Zhang, and D. J. Young, Corrosion Science 130, 161 (2018).
T. D. Nguyen, J. Zhang, D. J. Young, Corrosion Science 100, 448 (2015).
J. Zurek, D. J. Young, E. Essuman, M. Haensel, H. J. Penkalla, L. Niewolak, and W. J. Quadakkers, Materials Science & Engineering A 477, 259 (2008).
T. D. Nguyen, J. Zhang, and D. J. Young, Corrosion Science 76, 231 (2013).
L. Niewolak, D. J. Young, H. Hattendorf, L. Singheiser, and W. J. Quadakkers, Oxidation of Metals 82, 123(2014).
H. Nagai and K. Obayashi, Journal of the American Ceramic Society 72, 400 (1989).
H. Nagai and M. Okabayashi, Transactions of the Japan Institute of Metals 22, 691 (1981).
V. B. Trindade, U. Krupp, Ph. E.-G. Wagenhuber, Y. M. Virkar, H.-J. Christ, Materials at High Temperatures 22, 207 (2005).
A. Safikhani, M. Esmailian, T. Tinatiseresht, and G. B. Darband, International Journal of Hydrogen Energy 41, 6045 (2016).
Y. Zhu, C. Li, Y. Liu, Z. Ma and H. Yu, Journal of Iron and Steel Research International 27, 1179 (2020)
D. Li, Y. Xu, and D. Lin, Journal of Materials Science 36, 979 (2001).
M. Abbasi, D.-I. Kim, J.-H. Shim, and W.-S. Jung, Journal of Alloys and Compounds 658, 210 (2016).
E. Essuman, L. R. Walker, P. J. Maziasz, and B. A. Pint, Materials Science and Technology 29(7), 822 (2013).
P. J. Ennis and W. J. Quadakkers, Corrosion and creep of nickel-base alloys in steam reforming gas, in High temperature alloys - Their exploitable potential, eds. J. B. Marriott. et al. , (Elsevier, London, 1987), p. 465
A. Holt and P. Kofstad, Solid State Ionics 117, 21 (1999).
S. Cruchley, H. E. Evans, M. P. Taylor, M. C. Hardy, and S. Stekovic, Corrosion Science 75, 58 (2013).
G. J. Yurek, K. Przybylski, C. M. Cotell, Y. K. Kim, Segregation of foreign ions to grain boundaries in chromium sesquioxide and aluminum oxide scales, Proceedings - Electrochemical Society 88-5 (1988), 286-295.
A. Vayyala, I. Povstugar, D. Naumenko, W. J. Quadakkers, H. Hattendorf, and J. Mayer, Journal of the Electrochemical Society 167, 061502 (2020).
A. S. Nagelberg, Oxidation of Metals 17, 415 (1982).
Y. Inoue, N. Hiraide, A. Hayashi, and K. Ushioda, Materials Transactions 60, 1968 (2019).
A. Karabela, L. G. Zhao, J. Tong, N. J. Simms, J. R. Nicholls, and M. C. Hardy, Materials Science & Engineering A 528, 6194 (2011).
K. El-Menshawy, H. P. Buchkremer, F. Tietz, and D. Stoever, Journal of Materials Science & Technology 22, 245 (2006).
J. D. Tucker, B. Adam, M. Anderson, B. Pint, G. R. Holcomb, C. S. Carney, H. Saari, , L. Teeter, J. Mahaffey, Ö. Doğan, C. Jang, S. Kung, Supercritical CO2 round robin test program, in The 6th International Supercritical CO2 Power Cycles Symposium, 2018, Pittsburgh, PA, USA Paper #146.
A. Erdogan, K. M. Doleker, and S. Zeytin, JOM 71, 3499 (2019).
T. P. Dudziak, K. Jahns, D. Wilk Kolodziejczyk, U. Krupp, A. Polkowska, V. Deodeshmukh, and M. Warmuzek, Advanced Engineering Materials 21, 1801142 (2019).
T. D. Nguyen, J. Zhang, and D. J. Young, Oxidation of Metals 87, 541 (2017).
B. A. Pint and K. A. Unocic, Oxidation of Metals 87, 515 (2017).
T. D. Nguyen, J. Zhang, and D. J. Young, Oxidation of Metals 81, 549 (2014).
G. Bamba, Y. Wouters, A. Galerie, F. Charlot, and A. Dellali, Acta Materialia 54, 3917 (2006).
M. Takeda, H. Kushida, T. Onishi, M. Toyama, F. Koizumi, and S. Fujimoto, Oxidation of Metals 73, 1 (2010)
D. J. Young and J. Zhang, JOM 70, 1493 (2018).
W. Assassa and P. Guiraldenq, Metal Science 12, 123 (1978).
Acknowledgements
The authors would like to thank M. Howell, M. Stephens, T. M. Lowe, T. Jordan, and V. Cox for assistance with the experimental work. J. Jun and R. Pillai provided helpful comments on the manuscript. This work has been carried out within the Academy of Finland project “Novel Approaches to Study Corrosion Mechanisms in High-temperature Industrial Processes” (Decision No. 296435). This research also was sponsored by the US Department of Energy, Office of Fossil Energy, Crosscutting Technology Program. ToF-SIMS characterization was conducted at the Center for Nanophase Materials Sciences, which is a DOE Office of Science User Facility, and using instrumentation within ORNL's Materials Characterization Core provided by UT-Battelle, LLC under Contract No. DE-AC05-00OR22725 with the US Department of Energy.
Funding
Open access funding provided by Abo Akademi University (ABO).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This manuscript has been authored by UT-Battelle, LLC under Contract No. DE-AC05-00OR22725 with the US Department of Energy. The United States Government retains and the publisher, by accepting the article for publication, acknowledges that the United States Government retains a non-exclusive, paid-up, irrevocable, world-wide license to publish or reproduce the published form of this manuscript, or allow others to do so, for United States Government purposes. The Department of Energy will provide public access to these results of federally sponsored research in accordance with the DOE Public Access Plan (https://www.energy.gov/downloads/doe-public-access-plan).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lehmusto, J., Ievlev, A.V., Cakmak, E. et al. A Tracer Study on sCO2 Corrosion with Multiple Oxygen-Bearing Impurities. Oxid Met 96, 571–587 (2021). https://doi.org/10.1007/s11085-021-10071-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11085-021-10071-6