Abstract
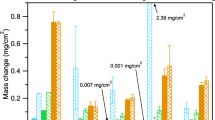
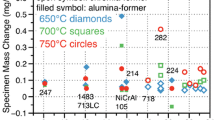
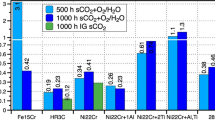
Future technologies require structural materials resistant to environmental degradation in high-temperature CO2-rich environments. Herein, we exposed several commercially available Ni-based alloys (230, 263, 282, 617, 625, and 740H) to atmospheric pressure gases intended to simulate the compositions expected in future direct-fired supercritical CO2 power cycles. The alloys were exposed to 95% CO2 + 4% H2O + 1% O2 and the same gas containing 0.1% SO2 at temperatures of 600, 650, 700, 750, and 800 °C for 2500 h. With minor exceptions, chromia scales formed on all alloys at all temperatures in the SO2-free gas, yielding parabolic growth rates that followed an Arrhenius temperature dependence. Behavior in the SO2-containing gas was more complex. Generally, the alloys performed well at temperatures of 650, 750, and 800 °C. While some alloys further performed relatively well across the whole temperature range, several of the alloys experienced chromia failure resulting in non-protective duplex oxide scales and high oxidation rates, at temperatures of 600 and 700 °C. Deviation from protective behavior was associated with internal sulfide formation and, additionally for the case of 600 °C, external sulfate formation. Extensive carburization accompanied growth of the non-protective duplex oxide scales, which made it more difficult for the alloy to recover after initiation of the sulfur-induced accelerated corrosion process. The thermodynamic and kinetic factors influencing the accelerated corrosion in the presence of sulfur are discussed. The results suggest that caution is required when assessing compatibility of Ni-based alloys for CO2-based systems when sulfur-based impurities are expected.











Similar content being viewed by others
References
K. Brun, P. Friedman, and R. Dennis, Fundamentals and Applications of Supercritical Carbon Dioxide (sCO2) Based Power Cycles, (Woodhead Publishing, 2017).
R. Allam, et al., Energy Procedia 114, 5948 (2017).
R. P. Oleksak and F. Rouillard, Materials performance in CO2 and supercritical CO2. in Comprehensive Nuclear Materials, 2nd ed, eds. R. J. M. Konings and R. E. Stoller (Elsevier, Oxford, 2020), p. 422. https://doi.org/10.1016/B978-0-12-803581-8.11622-4
R. P. Oleksak, G. R. Holcomb, S. Carney, and Ö. N. Doğan, Corrosion Science 206, 110488 (2022).
B. A. Pint, R. Pillai, M. J. Lance, and J. R. Keiser, Oxidation of Metals 94, 505 (2020).
R. P. Oleksak, J. H. Tylczak, G. R. Holcomb, and Ö. N. Doğan, Corrosion Science 157, 20 (2019).
B. A. Pint, R. G. Brese, and J. R. Keiser, The Effect of Impurities and Pressure on Oxidation in CO2 at 700°–800 °C, in 2018 NACE CORROSION Conference (NACE International, Phoenix).
P. Huczkowski, D. J. Young, T. Olszewski, A. Chyrkin, and W. J. Quadakkers, Oxidation of Metals 89, 651 (2018).
N. Mu, K. Y. Jung, N. M. Yanar, G. H. Meier, F. S. Pettit, and G. R. Holcomb, Oxidation of Metals 78, 221 (2012).
J. Mahaffey, et al., Metallurgical and Materials Transactions A 49, 3703 (2018).
J. Mahaffey, D. Adam, A. Brittan, M. Anderson, and K. Sridharan, Oxidation of Metals 86, 567 (2016).
J. Lehmusto, J. Kurley, M. Lance, J. Keiser, and B. A. Pint, Oxidation of Metals 94, 95 (2020).
B. A. Pint, J. Lehmusto, M. J. Lance, and J. R. Keiser, Materials and Corrosion 70, 1400 (2019).
K. Li, Y. Zeng, and J.-L. Luo, Corrosion Science 184, 109350 (2021).
J. P. Shingledecker, S. C. Kung, and I. G. Wright, Predicting the Oxidation/Corrosion Performance of Structural Alloys in Supercritical CO2, (Electric Power Research Institute, 2017), p. 1.
R. P. Oleksak, C. S. Carney, and Ö. N. Doğan, Corrosion Science 215, 111055 (2023).
R. P. Oleksak, J. H. Tylczak, C. S. Carney, G. R. Holcomb, and Ö. N. Doğan, JOM Journal of the Minerals Metals and Materials Society 70, 1527 (2018).
R. P. Oleksak, J. H. Tylczak, G. R. Holcomb, and O. N. Dogan, JOM Journal of the Minerals Metals and Materials Society 72, 1822 (2020).
M. Stroosnijder and W. Quadakkers, High Temperature Technology 4, 141 (1986).
F. Gesmundo, D. Young, and S. Roy, High Temperature Materials and Processes 8, 149 (1989).
H. J. Grabke, R. Lobnig, and P. Papaiacovou, Mechanisms of oxidation and sulfidation of high temperature alloys in H2-H2O-H2S mixtures. in Selected Topics in High Temperature Chemistry: Defect Chemistry of Solids, eds. Ø. Johannesen and A. G. Andersen (Elsevier, New York, 1989), p. 1989.
J. Stringer, Mixed oxidant corrosion in coal combustion and conversion systems: manifestations and mechanisms, in High-Temperature Oxidation and Sulphidation Processes (Elsevier, 1990), p. 257.
T. Gheno and B. Gleeson, Oxidation of Metals 84, 567 (2015).
B. Grégoire, X. Montero, M. C. Galetz, G. Bonnet, and F. Pedraza, Corrosion Science 155, 134 (2019).
R. P. Oleksak, C. S. Carney, G. R. Holcomb, and Ö. N. Doğan, Oxidation of Metals 90, 27 (2017).
R. P. Oleksak, M. Kapoor, D. E. Perea, G. R. Holcomb, and Ö. N. Doğan, NPJ Materials Degradation 2, 25 (2018).
A. Chyrkin, P. Huczkowski, V. Shemet, L. Singheiser, and W. J. Quadakkers, Oxidation of Metals 75, 143 (2011).
D. Caplan and G. Sproule, Oxidation of Metals 9, 459 (1975).
J. H. Chen, P. M. Rogers, and J. A. Little, Oxidation of Metals 47, 381 (1997).
B. Gleeson, Materials Research 7, 61 (2004).
R. Peraldi, D. Monceau, and B. Pieraggi, Oxidation of Metals 58, 275 (2002).
C. Sha, L. Yang, J. M. Cairney, J. Zhang, and D. J. Young, Corrosion Science 222, 111410 (2023).
M. C. Pope and N. Birks, Oxidation of Metals 12, 173 (1978).
N. Birks, Corrosion mechanisms of metals and alloys in multicomponent oxidative environments, in Symposium on Properties of High Temperature Alloys with Emphasis on Environmental Effects, Las Vegas, NV, USA, eds. Z. A. Foroulis and F. S. Pettit (Electrochemical Society), p. 215.
S.-H. Choi and J. Stringer, Materials Science and Engineering 87, 237 (1987).
Z.-S. Liu, W.-K. Li, and M.-J. Hung, Journal of the Air & Waste Management Association 64, 1038 (2014).
B. Li and C. Ma, Energy Procedia 153, 471 (2018).
M. H. LaBranche and G. J. Yurek, Oxidation of Metals 28, 73 (1987).
Acknowledgements
This work was performed in support of the U.S. Department of Energy’s Fossil Energy and Carbon Management Advanced Energy Materials Research Program. The Research was executed through the National Energy Technology Laboratory Research and Innovation Center’s Advanced Materials Development Field Work Proposal. We thank Trevor Godell (NETL) for machining the samples, Christopher McKaig (NETL) and Matthew Fortner (NETL) for metallographic preparation of the sample cross-sections, Keith Collins (NETL) for performing EPMA analysis, and Richard Chinn (NETL) for performing XRD analysis.
Funding
This work was funded by the U.S. Department of Energy, National Energy Technology Laboratory, an agency of the United States Government. Neither the United States Government nor any agency thereof, nor any of their employees, makes any warranty, expressed or implied, or assumes any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product, or process disclosed, or represents that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process, or service by trade name, trademark, manufacturer, or otherwise, does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States Government or any agency thereof. The views and opinions of authors expressed herein do not necessarily state or reflect those of the United States Government or any agency thereof.
Author information
Authors and Affiliations
Contributions
R.O. performed characterization and thermodynamic analyses, interpreted the results, prepared the figures, and conceived of and wrote the manuscript. J.T. performed the oxidation experiments. L.T. and C.C. performed additional characterization. O.D. proposed the research and provided guidance throughout the project. All authors contributed to discussion of the results and editing of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Oleksak, R.P., Tylczak, J.H., Teeter, L. et al. High-Temperature Corrosion of Chromia-Forming Ni-Based Alloys in CO2 Containing Impurities. High Temperature Corrosion of mater. 100, 597–620 (2023). https://doi.org/10.1007/s11085-023-10189-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11085-023-10189-9