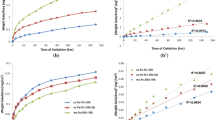
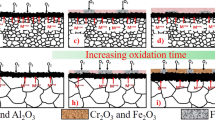
From the 1980’s a theory named “the sulfur effect” has been applied to explain the scale adhesion and the reactive-element effect (REE) during high-temperature oxidation. It claims that the bond between the oxide scale and the metal substrate is intrinsically strong and that impurity sulfur in the metal segregates at the oxide scale/substrate interface and weakens the bond, and that REs getter the sulfur impurity and prevent it from segregating to the interface. In the present study, a cast polycrystalline sulfur-containing Fe–25Cr–5Al-1S (wt.%) alloy and its magnetron-sputtered nanocrystalline coating were oxidized at 1000°C, and the specimens were examined by XRD and SEM. The scale formed on the cast alloy was cracked and detached from the substrate even after isothermal exposure, and obvious sulfur enrichment was detectable at the scale/substrate interface. While, the scale formed on the nanocrystalline coating was very adherent after 100 cycles oxidation. Here, sulfur was preferentially distributed in the outer scale and internal oxides rather than at the scale/substrate interface. These results provide evidence that nanocrystallization can prevent sulfur segregation at the scale/substrate interface, hence enhance scale adhesion.







Similar content being viewed by others
References
Allam I. M., Whittle D. P., and Stringer J., (1978). Oxidation of Metals 12: 35
Stott F. H., Golightly F. A., and Wood G. C., (1979). Corrosion Science 19: 889
Tien J. K., and Pettit F. S., (1972). Metallurgical Transactions 3: 1587
Whittle D. P., and Stringer J., (1980). Philosophical Transactions of the Royal Society of London Series A 285: 309
Stott F. H., and Wood G. C., (1987). Material Science Engineering 87: 261
Huntz A. M., (1987). Material Science Engineering 87: 251
Hou P. Y., and Stringer J., (1995). Material Science Engineering A202: 1
Smeggil J. G., Funkenbusch A. W., and Bornstein N. S., (1986). Metallurgical Transactions A17: 923
Lees D. G., (1987). Oxidation of Metals 27: 75
Smeggil J. G., (1987). Material Science Engineering 87: 261
P. Y. Hou, and J. Stringer, in Microscopy of Oxidation. M. J. Bennett, and G. W. Lorimer, eds. (The Inst. of Metals, 1991), pp. 362–368
Hou P. Y., and Stringer J., (1992). Oxidation of Metals 38: 323
Grabke H. J., Wiemer D., and Viefhaus H., (1991). Applied Surface Science 47: 243
Tolpygo V. K., and Viefhaus H., (1999). Oxidation of Metals 52: 1
Hou P. Y., and Ackerman G. D., (2001). Applied Surface Science 178: 156
Hou P. Y., Zhang X. F., and Cannon R. M., (2004). Scripta Materialia 50: 45
Hou P. Y., (2000). Materials and Corrosion 51: 329
Smialek J. L., (1987). Metallurgical Transactions 18A: 164
Smialek J. L., (1991). Metallurgical Transactions 22A: 739
Smialek J. L., and Tubbs B. K., (1995). Metallurgical and Materials Transactions 26A: 427
Smialek J. L., Jayne D. T., Schaeffer J. C., and Murphy W. C., (1994). Thin Solid Films 253: 285
Smialek J. L., (2001). Oxidation of Metals 55: 75
Smialek J. L., (1998). Transactions of the ASME 120: 370
Lou H. Y., and Wang F. H., (1992). Vacuum 43: 757
Lou H. Y., Zhu S. L., and Wang F. H., (1995). Oxidation of Metals 43: 317
Wang F. H., Lou H. Y., and Wu W. T., (1995). Oxidation of Metals 43: 395
Wang F. H., Lou H. Y., Zhu S. L., and Wu W. T., (1996). Oxidation of Metals 45: 39
Wang F. H., (1997). Oxidation of Metals 48: 215
Wang F. H., (1997). Oxidation of Metals 47: 247
Yang S. L., Wang F. H., Wu W. T., (2001). Intermetallics 9: 741
Peng X., Wang F. H., (2003). Corrosion of Science 45: 2293
Chen G. F., Lou H. Y., (2000). Oxidation of Metals 53: 467
Giggins C. S., and Pettit F. S., (1969). Transactions of the Metallurgical Society of AIME 245: 2495
Giggins C. S., and Pettit F. S., (1969). Transactions of the Metallurgical Society of AIME 245: 2509
Merz M. D., (1979). Metallurgical Transactions 10A: 71
Le Gall R., Querard E., Saindrenan G., Mourton H., and Roptin D., (1996). Scripta Materialia 35: 1175
Quadakkers W. J., Wasserfuhr C., Khanna A. S., and Nickel H., Material Sciences and Technology 4: 1119 (1988)
Khanna A. S., Jonas H., and Quadakkers W. J., Werkstoffe und Korrosion 40: 552 (1989)
J. L. Smialek, in Procedings of High Temp. Materials Chemistry IV, Z. A. Munir, D. Cubicciotti, and H. Tagawa, eds (Electrochem. Soc., 1998) pp. 241–253
Smialek J. L., (2000). Meterials at High Temperature 17: 71
Amano T., Watanabe T., and Michiyama K., (2000). Oxidation of Metals 53: 451
Yang S. L., Wang F. H., Sun Z. M., Zhu S. L., (2002). Intermetallics 10: 467
Fox P., Lees D. G., and Lorimer G. W., (1991). Oxidation of Metals 36: 491
Hahn H., Hofler J. H., and Averback R. S., (1989). Defect and Diffusion Forum 66–69: 549
Acknowledgment
This work is supported by the National Natural Scientific Foundation of China (NSFC).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, S., Wang, F. Effect of Nanocrystallization on Sulfur Segregation in Fe–Cr–Al Alloy during Oxidation at 1000°C. Oxid Met 65, 195–205 (2006). https://doi.org/10.1007/s11085-006-9014-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11085-006-9014-1