Abstract
Origin of life processes might have begun with the formation of important biomonomers, such as amino acids and nucleotides, from simple molecules present in the prebiotic environment and their subsequent condensation to biopolymers. While studying the prebiotic synthesis of naturally occurring purine and pyrimidine derivatives from formamide, the manganese oxides demonstrated not only good binding for formamide but demonstrated novel catalytic activity. A novel one pot manganese oxide catalyzed synthesis of pyrimidine nucleobases like thymine is reported along with the formation of other nucleobases like purine, 9-(hydroxyacetyl) purine, cytosine, 4(3 H)-pyrimidinone and adenine in acceptable amounts. The work reported is significant in the sense that the synthesis of thymine has exhibited difficulties especially under one pot conditions and also such has been reported only under the catalytic activity of TiO2. The lower oxides of manganese were reported to show higher potential as catalysts and their existence were favored by the reducing atmospheric conditions prevalent on early Earth; thereby confirming the hypothesis that mineral having metals in reduced form might have been more active during the course of chemical evolution. Our results further confirm the role of formamide as a probable precursor for the formation of purine and pyrimidine bases during the course of chemical evolution and origin of life.
Similar content being viewed by others
Introduction
Literature study has established the interplay of several hypothetical processes leading to the emergence of life (Oparin 1957). Bio monomers like amino acids and nucleotides are known to have developed from simple organic molecules like HCN, HCHO, HCONH2 etc. which existed in the prebiotic environment and ultimately are known to have condensed to form stable bio-molecules (Miller 1953). Essential prerequisites for such processes leading to the origin of life were suggested to be the involvement of chemically stable compounds and a stable micro-environment (Saladino et al. 2001). HCN is considered as a key precursor in the synthesis of nitrogen-containing biomolecules on the primitive Earth (Miller 1955; Abelson 1966). The pioneering work demonstrating the synthesis of adenine from HCN (Oró 1960; Oró and Kamat 1961; Oró and Kimball 1961) was followed by decades of study on the HCN chemistry [as reviewed in (Orgel 2004) and (Delaye and Lazcano 2005)]. But two main issues remain unresolved in the HCN chemistry: (i) the thermodynamic instability of HCN under hydrolytic conditions and (ii) the narrow panel of nucleobases, limited only to purines that can be formed via HCN condensation processes. Hence the possibility of the role of formamide in the synthesis of nucleobases was initiated and further established by the work of Bredereck et al. and Yamada (Bredereck et al. 1959; Yamada and Okamoto 1972; Yamada et al. 1978a, b). More recently the synthesis of a large variety of both purine and pyrimidine nucleic bases, of some of their precursors and of their degradation products from formamide was reported (Saladino et al. 2005, 2007, 2009; Ciciriello et al. 2009). Such products were obtained simply by heating formamide between 100 and 160 °C in the presence of one or more minerals that were known to exist in the prebiotic environment e.g. silica, alumina, zeolites, CaCO3, cosmic dust, clays, kaolin, montmorillonites, olivines, phosphate minerals (Saladino et al. 2001, 2005, 2007), sulphur and iron minerals (Saladino et al. 2008), clay, zirconium-based (Saladino et al. 2010), and boron-based (Saladino et al. 2011) minerals. Guanine, which is missing from the compounds obtained in heat treatment of formamide is presumably because of its instability, but can be obtained from formamide by UV irradiation (Barks et al. 2010) at lower temperatures.
Also, the role of different minerals and metal oxides acting as catalyst in the formation of nucleobases cannot be underestimated. These compounds act as catalysts for the condensation of formamide to biomolecules, or alternatively, they can control the degradation kinetics of formamide to other compounds which are useful intermediates for prebiotic syntheses (Saladino et al. 2008). Moreover, minerals are able to preserve newly synthesized biomolecules from chemical and photochemical degradations (Gallori et al. 2006; Scappini et al. 2004). Kobayashi studied the importance of transition metals in chemical evolution (Kobayashi and Ponnamperuma 1985a, b, Kobayashi and Haraguchi 1988). Recently, various studies have amply demonstrated that transition metals and their oxides like hematite (Arora et al. 2007), zinc oxide (Arora and Kamaluddin 2007), aluminum oxide (Arora and Kamaluddin 2009), iron oxides (goethite, akaganeite and hematite) (Uma Shanker et al. 2011) had good catalytic activity in the formation of several nucleobases from formamide and hence suggested their role in the prebiotic synthesis.
On a similar line of thought, it is seen that manganese is the tenth most abundant element in the biosphere (Turekian and Wedepohl 1961; Heiserman 1992) and its existence on Mars has also been reported (Heiserman 1992). Its involvement in the catalysis of formation of oligomers (Visscher and Schwartz 1989; Hroacki and Hiromichi 1999) provides a pathway for its possible role in the molecular evolution of life on Earth. But manganese is known to exist in various oxidation states on Earth. The early Earth had a predominantly reducing atmosphere as proposed by pioneering works of Oparin and further supported by works of Chyba and Sagan (Chyba and Sagan 1992). Thus, under such conditions, manganese oxides of lower oxidation states would have been prevalent with volcanoes being their major source. Higher oxides of manganese may have been formed by the microbial oxidation of the soluble Mn (II) in natural environments (Nealson et al. 1988). In a review by Hazen et al., (Hazen et al. 2008) it was pointed out that the marine sediments deposited in South Africa, Gabon, and India have high levels of manganese as carbonates, oxides etc. and constitute large economic deposits of minerals such as kutnahorite [Ca(Mn,Mg,Fe)(CO3)2], pyrolusite (MnO2), rhodochrosite (MnCO3), rhodonite [(Mn,Fe,Ca)SiO3], bixbyite [(Mn,Fe)2O3], braunite (Mn2 +Mn6 3+SiO12), and hausmannite (Mn2 +Mn2 3+O4) (Leclerc and Weber 1980; Dasgupta et al. 1992; Tsikos and Moore 1997; Roy 2006). This confirms that higher oxides of manganese were not formed until the Paleoproterozoic Era (2.5 to 1.9 Ga). In order to elucidate the involvement of the lower as well as the higher oxides of manganese on the prebiotic synthesis of nucleobases from formamide, we synthesized different forms of manganese oxides namely, manganosite (MnO), bixbyite (Mn2O3), hausmannite (Mn3O4) and pyrolusite (MnO2) as potential representative prebiotic models of catalysts involved in chemical evolution. Such oxides thus could aid in the catalysis of reactions like synthesis of nucleobases from formamide thereby paving a path for the evolution of the early life. Although in our earlier work (Bhushan et al. 2011), it was established that manganese in the lower oxidation state had the potential to aggregate and entrap organic polymers like ribonucleotides; still studies were undertaken on all oxides of manganese in order to make a comparative study and further make confirmations on existing facts.
Experimental Procedure
Material and Methods
Manganese acetate (Merck), Ammonium oxalate (Merck), potassium dihydrogen phosphate (Merck), orthophosphoric acid (Merck), formamide (>99.6 %), adenine, cytosine, purine, thymine, 4(3 H)-pyrimidinone were purchased from Sigma. All other chemicals used were of an analytical grade and were used without further purification. Millipore water was used throughout the studies.
Synthesis of Nucleobases from Formamide
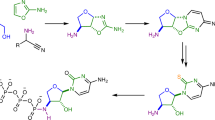
In this experiment, the upper limit of temperature of 180 °C for the synthesis of nucleobases from formamide was set in accordance with the boiling point of formamide (211 °C). The lowest temperature at which formamide (1) undergoes appreciable thermal decomposition is reported to be 180-190 °C (Kirkpatrick, 1944). Thus the most favorable set of condition for synthesis of nucleobases from formamide was a high concentration of formamide, the presence of catalytic system and a temperature range of 100-180 °C (Saladino et al. 2001). The reaction was carried out in a round bottom flask by taking formamide (1) (5.7 g, 5 ml, 0.12 mol) in the temperature range 100–160 °C for 12–96 h in the presence of 50 mg of selected catalyst manganosite (MnO), bixbyite (Mn2O3), hausmannite (Mn3O4) and pyrolusite (MnO2). A blank experiment (absence of manganese oxides) was also performed under similar reaction conditions. The reaction mixture was centrifuged and filtered through 0.2 μm filter paper and then the mixture was divided into two parts, one part was used for HPLC analysis and the other for ESI-MS analysis. It was observed that the product gradually appeared after 12 h and concentration of the products became constant after 48 h thus the optimum time for the formation of the products was approximately 48 h. Low yield of the products was observed below 160 °C. Our attention was mainly focused on the formation and identification of purine and pyrimidine derivatives at 160 °C where relatively high yield was observed. The main identified products were purine (2), adenine (3), cytosine (4), 9-(hydroxyacetyl) purine (5), thymine (6) and 4(3 H)-pyrimidinone (7) (Scheme 1). Only purine (2) was formed in the blank experiment.
High-performance Liquid Chromatography Analysis:
All the solutions obtained from the reaction system were analyzed with an Agilent 1100 series LC system using an Agilent Hypersil (ODS 5 μm/200 × 2.2 cm) column. The mobile phase used was a buffer solution of (KH2PO4 + H3PO4) of pH ~ 4.05; with a flow rate of 1.00 ml/min under isocratic condition at 260 nm.
The HPLC chromatograms showed a number of peaks including purines and pyrimidines in all the reaction mixtures. The products identified were purine (2), adenine (3), cytosine (4), 9-(hydroxyacetyl) purine (5), thymine (6) and 4(3 H)-pyrimidinone (7), which were confirmed by co-injection with authentic samples. It was noted that all the four manganese oxides produced nearly the same number of products with different % yield. Manganosite (MnO) has shown the highest yield purine (2), adenine (3), cytosine (4), 9-(hydroxyacetyl) purine (5), thymine (6) and 4(3 H)-pyrimidinone (7). Hausmannite (Mn3O4) also showed a higher yield of purine compared to bixbyite (Mn2O3) but other products were in lesser yield. Pyrolusite (MnO2) afforded the lowest yield of the products in comparison to other manganese oxides. The yield obtained was measured in mg of product formed per gram of formamide with the help of standard curve.
Electro spray Ionization–Mass Spectrometry Analysis:
A Bruker Esquire 4000 (Bruker Daltonics Data Analysis 3.3, Germany) ion trap mass spectrometer interfaced to electro spray ionization (ESI) source was used for mass analysis of nucleobases formed from formamide in the presence of manganese oxides. Ionization of analytes was carried out using the following setting of ESI: nebulizer gas flow 10 psi, dry gas 5 L min−1, dry temperature 300 °C, capillary voltage 4000 V. Calibration of m/z was performed using ES-tuning mix. The ESI-MS/MS experiments of the products were also performed under the same conditions using positive ionization mode.
Results
Studies on the formation of nucleobases from formamide (1) showed that in absence of catalysts only purine (2) was formed, while in the presence of manganosite (MnO), bixbyite (Mn2O3), hausmannite (Mn3O4) and pyrolusite (MnO2) formamide (1) afforded a number of various nucleobases namely, purine (2), adenine (3), cytosine (4), 9-(hydroxyacetyl) purine (5), thymine (6) and 4(3 H)-pyrimidinone (7) and such were obtained in high yield (Scheme 1, Table 1).
The identification of the products were carried out by HPLC and further confirmed by electro spray ionization mass spectrometry (ESI- MS) technique. The yields were recorded in mg of product formed per gram of formamide. The HPLC results (Fig. 1) showed number of peaks including purines and pyrimidines after analysis of all the reaction mixtures. The products identified were purine purine (2), adenine (3), cytosine (4), 9-(hydroxyacetyl) purine (5), thymine (6) and 4(3 H)-pyrimidinone (7), which were confirmed by co-injection with authentic samples. Prepared solutions of at least five different concentrations of each standard compound were used to construct standard calibration curves. Spiking of reaction products with standard compounds was also performed to confirm retention time. It was observed that all the forms of manganese oxides used produced nearly the same products, with different % yield. Manganosite (MnO) was found to produce the highest yield of purine (2), adenine (3), cytosine (4), 9-(hydroxyacetyl) purine (5), thymine (6) and 4(3 H)-pyrimidinone (7) from formamide (1). Presence of hausmannite (Mn3O4) also produced a lower yield of purine compared to manganosite (MnO) and other products were obtained in lesser yield. Bixbyite (Mn2O3) and pyrolusite (MnO2) afforded the lowest yield of the products in comparison to other manganese oxides. It was observed that formation of product gradually started after 12 h and yield of the products became constant after 48 h (Fig. 2). Low yield of the products were observed below 150 °C.
The mass spectra of the reaction mixtures obtained were characterized by [M + nH] + ions. Analysis of the reaction solutions by ESI-MS showed formation of cytosine (4) [M+ H]+ m/z 112, 4(3 H)-pyrimidinone (7) [M+ H]+ m/z 97, purine (2) [M+ H]+ m/z 121, 9-(hydroxyacetyl) purine (5) [M + H]+ 179, adenine (3) [M+ H]+ m/z 136, thymine (6) [M+ H]+ m/z 127 and adenine (3) [M+ 3 H]+ m/z 139. Figure 3 illustrates the ESI MS analysis results of the products formed by heating formamide with manganosite (MnO), bixbyite (Mn2O3), hausmannite (Mn3O4) and pyrolusite (MnO2). As shown in Fig. 3 the peaks at m/z 97, 112, 121, 179, 136, 127 and 139 etc. were identified as protonated 4(3 H)-pyrimidinone (7), cytosine (4), purine (2), 9-(hydroxyacetyl) purine (5), thymine (6) and adenine (3), respectively.
Discussion
The results here suggest that the synthesis of thymine (6)-a pyrimidine nucleobase is possible under the catalytic activity of manganese oxides via its thermal treatment. The findings are novel in the sense that synthesis of thymine from formamide using transition metal oxides as catalyst has not been reported earlier. Previous synthesis of thymine in thermal conditions from formamide was reported only in the case of TiO2, requiring the hydroxymethylation of uracil as key step (Saladino et al. 2003). Other possible routes of synthesis of thymine have been reported in literature via formation of uracil from dehydration of dihydrouracil and subsequent addition of acetate to uracil (Chittenden and Schwartz 1976, Schwartz and Chittenden 1977). Thymine was also synthesized by Choughuley et al. 1977 via treatment of uracil with formaldehyde and formic acid in aqueous solution at 140 °C. Stephen et al. synthesized thymine by methylation of uracil with formaldehyde and hydrazine (Stephen-Sherwood et al. 1971).
The results further suggest that manganese oxides are efficient catalysts for the formation of nucleobases from formamide. Saladino found the same products in the presence of different minerals (Saladino et al. 2001). The significance of the above study is that manganosite (MnO) and hausmannite (Mn3O4) afford almost the same yield of purine (2) while hausmannite (Mn3O4) affords lower yield under the same conditions of the experiment. The surface area of manganosite (MnO) is 238.89 m2/g whereas those for hausmannite (Mn3O4), bixbyite (Mn2O3) and pyrolusite (MnO2) are 226.56 m2/g, 218.35 m2/g and 214.57 m2/g respectively (SI Table 1). Since the surface area of manganosite (MnO) is nearly similar to that of hausmannite (Mn3O4), therefore it seems to be reasonable that the yield of the products could not be explained only on the basis of surface area of the catalysts but some other factors such as structural shape and surface acidity maybe are playing a pivotal role (Yao and Millero, 1996; Daou et al., 2007). In view of these results it is proposed that manganese oxides present on the primitive Earth (Turekian and Wedepohl 1961) could have played a significant role in the synthesis of various biologically important compounds (Hroacki and Hiromichi 1999, Visscher and Schwartz 1989). These catalysts might have increased the thermal stability of nucleobases through molecular recognition processes or by simply isolating them from the external environmental conditions.
The early Earth conditions having a reducing nature as proposed by various researchers (Oparin 1938; Urey 1952; Miller 1953) favored the existence of manganese in its +2 and +3 oxidation states. Although all oxides of manganese showed catalytic activity, yet it can be stressed that it is the lower oxidation states which have provided their surfaces for adsorbing formamide molecules and which in turn have enhanced their catalytic role for the synthesis of nucleobases and other biologically important compounds (Jortner 2006; Saladino et al. 2008; Hroacki and Hiromichi 1999; Visscher and Schwartz 1989). But since the higher oxides of manganese had no existence until the Paleoproterozoic Era when the atmospheric conditions witnessed a rise in the oxygen levels, it can be concluded that manganese in its reduced form might have been more active during the course of chemical evolution.
Above study also supports the hypothesis that formamide is a probable precursor for the formation of purine and pyrimidine bases during the course of chemical evolution and origin of life.
Conclusion
The present work shows the success of synthesis of a major pyrimidine nucleobase- thymine (6) as a result of catalytic activity of manganese oxides from thermal treatment of formamide (1). This has major significance in the sense that no other transition metal oxide has been reported till date which acts as a catalyst for producing thymine (6) from the thermal treatment of formamide. The present study shows the importance of manganese oxides as prebiotic catalysts in chemical evolution and origin of life which might have played a significant role in the synthesis of small molecules like nucleobases and also in condensation of these small moieties into larger molecules which are ultimately responsible for the emergence of life on the earth. Among the various existing oxidation states of manganese, it is the manganosite (MnO) and hausmannite (Mn3O4) that have shown good catalytic activity due to their strong binding with formamide. The present study also supports the hypothesis that formamide is a probable precursor for the formation of purine and pyrimidine bases during the course of chemical evolution and origin of life.
Abbreviations
- ESI:
-
electro spray ionization
- HPLC:
-
high-performance liquid chromatography
- MS:
-
mass spectrometer, mass spectrometry
- MS/MS:
-
tandem mass spectrometer, tandem mass spectrometry
- m/z :
-
mass-to-charge ratio
References
Abelson PH (1966) Chemical events on the primitive earth. Proc Natl Acad Sci U S A 55:1365–1372
Arora AK, Kamaluddin (2007) Interaction of ribose nucleotides with zinc oxide and relevance in chemical evolution. Colloids and Surfaces a: Physicochemical and Engineering Aspect 298:186–191
Arora AK, Kamaluddin (2009) Role of metal oxides in chemical evolution: interaction of alumina with ribose nucleotides. Astrobiology 9:165–171
Arora AK, Tomar V, Aarti VKT, Kamaluddin (2007) Haematite–water system on Mars and its possible role in chemical evolution. Int. J. Astrobiology 6:267–271
Barks H, Buckley R, Grieves GA, Di Mauro E, Hud N, Orlando T (2010) Guanine, adenine, and hypoxanthine production in UV-irradiated formamide solutions: relaxation of the requirements for prebiotic purine nucleobase formation. Chembiochem 11:1240–1243
Bhushan B, Shanker U, Kamaluddin (2011) Adsorption of ribose nucleotides on manganese oxides with varied Mn/O Ratio: implications for chemical evolution. Orig Life Evol Biosph 41:469–482
Bredereck H, Gompper R, Schuh HGV, Theilig G (1959) Neuere methoden der preparativen organischen chemie II. 16. synthesen mit Säure-amiden, insbesondere mit formamid. Angew Chem 71:753–774
Chittenden GJF, Schwartz AW (1976) Prebiotic uracil synthesis by photodehydrogenation. Nature 263:350–351
Choughuley AS, Chada MS, Subbaraman AS (1977) A possible prebiotic synthesis of thymine: uracil-formaldehyde-formic acid reaction. Biosystems 1977(9):73–80
Chyba C, Sagan C (1992) Endogenous production, exogenous delivery and impact-shock synthesis of organic molecules: an inventory for the origins of life. Nature 355:125–132
Ciciriello F, Costanzo G, Pino S, Di Mauro E (2009) “Spontaneous generation revisited at the molecular level” in Evolutionary Biology: Concept, Modelling and application, 3–22 Ed. P. Pontarotti Springer Verlag Berlin Heidelberg
Daou TJ, Begin-Colin S, Greneche JM (2007) Phosphate Adsorption Properties of Magnetite-Based Nanoparticles. Chem Mater 19:4494--4505
Dasgupta S, Roy S, Fukuoka M (1992) Depositional models for manganese oxide and carbonate deposits of the Precambrian Sausar Group, India. Econ Geol 87:1412–1418
Delaye L, Lazcano A (2005) Prebiological evolution and the physics of the origin of life. Physics Life Rev 2:47–64
Gallori E, Biondi E, Branciamore S (2006) Looking for the primordial genetic honeycomb. Orig Life Evol Biosph 36:493–499
Hazen RM, Papineau D, Bleeker W, Downs Robert T, Ferrrry John M, McCoy Timothy J, Sverjrjensky Dimitri A, Yang H (2008) Mineral evolution. Am Mineral 93:1693–1720
Heiserman, David L (1992) Exploring Chemical Elements and their Compounds
Hroacki S, Hiromichi W (1999) Synthesis and reaction of nucleoside 5- diphosphate imidazolide: a nonenzymatic capping agent for 5-monophosphorylated oligoribonucleotides in aques solution. J. Org. Chem. 64:5836–5840
Jortner J (2006) Conditions for the emergence of life on the early earth: summary and reflections. Phil. Trans. R. Soc. B. 361:1877–1891
Kirkpatrick EC (1944) Stabilization of organic compounds (Assigned to E.I. Du Pont de Nemours & Co. Inc) U.S. Patent, 2:346--425
Kobayashi K, Haraguchi H (1988) Metalloenzymes in sea water–their significance in geochemistry and chemical evolution. Nihon Kaisui Gakkaishi 42:168
Kobayashi K, Ponnamperuma C (1985a) Trace elements in chemical evolution I. Orig. Life 15:55
Kobayashi K, Ponnamperuma C (1985b) Trace elements in chemical evolution II. Orig Life 16:67
Leclerc J, Weber F (1980) Geology and genesis of the moanda manganese deposits. In: Varentsov IM, Grasselly G (eds) Geology and geochemistry of manganese, 2. E. Schweizerbart’sche Verlagsbuchhandlung, Stuttgart, pp. 89–109
Miller SL (1953) A production of amino acids under possible primitive earth conditions. Science 117:528–529
Miller SL (1955) Production of some organic compounds under possible primitive earth conditions. J Am Chem Soc 77:2351–2361
Nealson KH, Tebo BM, Rosson RA (1988) Occurrence and mechanisms of microbial oxidation on manganese. Adv Appl Microbiol 33:279–318
Oparin AI (1938) The origin of life. Macmillan, New York
Oparin AI (1957) The origin of life on earth. Oliver & Boyd, Edinburgh
Orgel LE (2004) Prebiotic adenine revisited: eutectics and photochemistry. Orig Life Evol Biosph 34:61
Oró J (1960) Synthesis of adenine from ammonium cyanide. Biochem Biophys Res Commun 2:407–412
Oró J, Kamat SS (1961) Amino acid synthesis from hydrogen cyanide under possible primitive earth conditions. Nature 190:442–443
Oró J, Kimball AP (1961) Synthesis of purine under possible primitive earth conditions I. A denine from hydrogen cyanide. Arch Biochem Biophys 94:217–227
Roy S (2006) Sedimentary manganese metallogenesis in response to the evolution of the earth system. Earth-Sci Rev 77:273–305
Saladino R, Crestini C, Costanzo G, Negri R, Di Mauro E (2001) Possible prebiotic synthesis of purine, adenine, cytosine and 4(3 h)-pyrimidinone from formamide: implications for the origin of life. Bio org Med Chem 9(1249):1253
Saladino R, Ciambecchini U, Crestini C, Costanzo G, Negri R, Di Mauro E (2003) One-pot TiO2-catalyzed synthesis of nucleic bases and acyclonucleosides from formamide: implications for the origin of life. Chembiochem 4:514–521
Saladino R, Crestini C, Costanzo G, Di Mauro E (2005) On the prebiotic synthesis of nucleobases, nucleotides, oligonucleotides, pre-RNA and pre-DNA molecules. Top Curr Chem 259:29–68
Saladino R, Crestini C, Ciciriello F, Costanzo G, Di Mauro E (2007) Formamide chemistry and the origin of informational polymers. Chem Biodivers 4:694–720
Saladino R, Neri V, Crestini C, Costanzo G, Graciotti M, Di Mauro E (2008) Synthesis and degradation of nucleic acid components by formamide and iron sulfur minerals. J Am Chem Soc 130:15512–15518
Saladino R, Crestini C, Ciciriello F, Pino S, Costanzo G, Di Mauro E (2009) From formamide to RNA: the roles of formamide and water in the evolution of chemical information. Res Microbiol 160:441–448
Saladino R, Neri V, Crestini C (2010) Role of clays in the prebiotic synthesis of sugar derivatives from formamide. Philisophical Magazine 90:2329–2337
Saladino R, Barontini M, Cossetti C, Di Mauro E, Crestini C (2011) The effects of borate minerals on the synthesis of nucleic acid bases, amino acids and biogenic carboxylic acids from formamide. Orig Life Evol Biosph 41:317–330
Scappini F, Casadei F, Zambone R, Franchi M, Gallori E, Monti S (2004) Protective effect of clay minerals on adsorbed nucleic acid against UV radiation: possible role in the origin of life. Int. J. Astrobiology 3:17–19
Schwartz AW, Chittenden GJ (1977) Synthesis of uracil and thymine under simulated prebiotic conditions. Biosystems 9:87–92
Shanker U, Bhushan B, Bhattacharjee G, Kamaluddin (2011) Formation of nucleobases from formamide in the presence of iron oxides: implication in chemical evolution and origin of life. Astrobiology 11:3
Stephen-Sherwood E, Oro J, Kimball AP (1971) Thymine: a possible prebiotic synthesis. Science 173:446–447
Tsikos H, Moore JM (1997) Petrography and geochemistry of the Paleoproterozoic hotazel iron formation, Kalahari manganese field, South Africa: implications for Precambrian manganese metallogenesis. Econ Geol 92:87–97
Turekian KK, Wedepohl KL (1961) Distribution of the elements in some Major Units of the Earth's crust. Geol Soc Am Bull 72:275–192
Urey H (1952) On the early chemical history of the earth and the origin of life. Proceedings of the National Academy Of Sciences USA 38:351–363
Visscher J, Schwartz AW (1989) Manganese-catalyzed oligomerizations of nucleotide analogs. J Mol Evol 29:284–287
Yamada H, Okamoto T (1972) Polycyclic N-heterocyclic compounds XIV. reactions of methylpyridines with formamide. chem. Pharm Bull 20:623
Yamada H, Hirobe M, Higashiyama K, Takahashi H, Suzuki K (1978a) Reaction mechanism for purine ring formation as studied by 13C-15 n coupling. Tetrahedron Lett 42:4039
Yamada H, Hirobe M, Higashiyama K, Takahashi H, Suzuki K (1978b) Thermal fission and reformation of the carbon-nitrogen bond in formamide as studied by 13C-15 n coupling. J Am Chem Soc 100
Yao W, Millero FJ (1996) Adsorption of phosphate on manganese dioxide in seawater. Envir Sci Technol 30:536--541
Acknowledgments
We are thankful to Indian Space Research Organization (ISRO), Bangalore, India, for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bhushan, B., Nayak, A. & Kamaluddin Catalytic Role of Manganese Oxides in Prebiotic Nucleobases Synthesis from Formamide. Orig Life Evol Biosph 46, 203–213 (2016). https://doi.org/10.1007/s11084-015-9480-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11084-015-9480-z