Abstract
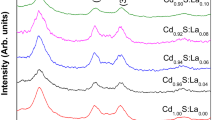
This study refers to the synthesis and characterization of a novel organic/inorganic hybrid nanocomposite material containing cadmium sulfide (CdS) nanoparticles. For this purpose, a series of polypropylene (PP)-g-polyethylene glycol (PEG), PP-g-PEG comb-type amphiphilic graft copolymers were synthesized. PEGs with Mn = 400, 2000, 3350, and 8000 Da were used and the graft copolymers obtained were coded as PPEG400, PPEG2000, PPEG3350, and PPEG8000. CdS nanoparticles were formed in tetrahydrofuran solution of PP-g-PEG amphiphilic comb-type copolymer by the reaction between aqueous solutions of Na2S and Cd(CH3COO)2 simultaneously. Micelle formation of PPEG2000 comb-type amphiphilic graft copolymer in both solvent/non-solvent (petroleum ether–THF) by transmission electron microscopy (TEM). The optical characteristics, size morphology, phase analysis, and dispersion of CdS nanoparticles embedded in PPEG400, PPEG2000, PPEG3350, and PPEG8000 comb-type amphiphilic graft copolymer micelles were determined by high resolution TEM (HRTEM), energy dispersive spectroscopy, UV–vis spectroscopy, and fluorescence emission spectroscopy techniques. The aggregate size of PPEG2000-CdS is between 10 and 50 nm; however, in the case of PPEG400-CdS, PPEG3350-CdS, and PPEG8000-CdS samples, it is up to approximately 100 nm. The size of CdS quantum dots in the aggregates for PPEG2000 and PPEG8000 samples was observed as 5 nm by HRTEM analysis, and this result was also supported by UV–vis absorbance spectra and fluorescence emission spectra.














Similar content being viewed by others
References
Alivisatos AP (1996) Semiconductor clusters, nanocrystals, and quantum dots. Science 271:5251
Balcı M, Allı A, Hazer B, Güven O, Cavicchi K, Çakmak M (2010) Synthesis and characterization of novel comb-type amphiphilic graft copolymers containing polypropylene and polyethylene glycol. Polym Bull 64:691–705
Biju V, Itoh T, Anas A, Sujith A (2008) Semiconductor quantum dots and metal nanoparticles: synthesis, optical properties, and biological applications. Anal Bioanal Chem 391:2469–2495
Brush L (1986) Electronic wave functions in semiconductor clusters: experiment and theory. J Phys Chem 90:2555–2560
Cao L, Miao Y, Zhang Z, Xie S, Yang G (2005) Exciton interactions in nanocrystal aggregates in reverse micelle. J Chem Phys 123:024702
Chestnoy N, Harris TD, Hull R, Brush LE (1986) Luminescence and photophysics of cadmium sulfide semiconductor clusters: the nature of the emitting electronic state. J Phys Chem 90:3393–3399
Chiu JJ, Kim BJ, Kramer EJ, Pine DJ (2005) Control of nanoparticle location in block copolymers. J Am Chem Soc 127:5036
Dong H, Hinestroza JP (2009) Metal nanoparticles on natural cellulose fibers: electrostatic assembly and in situ synthesis. ACS Appl Mater Interfaces 1:797–803
Erciyes AT, Erim M, Hazer B, Yağcı Y (1992) Synthesis of polyacrylamide flocculants with PEG segments by redox polymerization. Angew Macromol Chem 200:163–171
Farmer SC, Patent TE (2001) Photoluminescent polymer/quantum dot composite nanoparticles. Chem Mater 13:3920–3926
Ferrer JC, Castillo SA, Alonso JL, Fernandez de Avila S, Mallavia R (2009) Synthesis and characterization of CdS nanocrystals stabilized in polyvinyl alcohol–sodium polyphosphate. Mater Lett 63:638–640
Firth AV, Haggata SW, Khanna PK, Williams SJ, Allen JW, Magennis SW, Samuel IDW, Cole-Hamilton DJ (2004) Production and luminescent properties of CdSe and CdS nanoparticle–polymer composites. J Lumin 109:163–172
Hazer B (1995) Grafting on polybutadiene with macro or macromonomer initiator containing poly(ethylene glycol) units. Macromol Chem Phys 196:1945–1952
Hazer B (2010) Amphiphilic poly(3-hydroxy alkanoate)s: potential candidates for medical applications. Int J Polym Sci, Article Number: 423460. doi:10.1155/2010/423460
Hazer DB, Hazer B (2011) The effect of gold clusters on the autoxidation of poly(3-hydroxy 10-undecenoate-co-3-hydroxy octanoate) and tissue response evaluation. J Polym Res 18:251–262
Hazer DB, Hazer B, Dinçer N (2011) Soft tissue response to the presence of polypropylene-g-poly(ethylene glycol) comb-type graft copolymers containing gold nanoparticles. J Biomed Biotechnol. doi:10.1155/2011/956169
Hazer DB, Kilicay E, Hazer B (2012a) Poly(3-hydroxyalkanoate)s: diversification and biomedical applications. A state of the art review. Mater Sci Eng C 32:637–647
Hazer DB, Mut M, Dinçer N, Sarıbaş Z, Hazer B, Özgen T (2012b) The efficacy of silver-embedded polypropylene-grafted polyethylene glycol-coated ventricular catheters on prevention of shunt catheter infection in rats. Childs Nerve Syst. doi:10.1007/s00381-012-1729-5
Heinglein A (1989) Small-particle research: physicochemical properties of extremely small colloidal metal and semiconductor particles. Chem Rev 89:1861–1873
Herron N, Wang Y, Eckert YH (1990) Synthesis and characterization of surface-capped, size-quantized CdS clusters. Chemical control of cluster size. J Am Chem Soc 112:1322–1326
Jamali S, Iranizad ES, Shayesteh SF (2007) Synthesis, optical and structural characterization of CdS nanoparticles. Int J Nanosci Nanotechnol 3(1):53–62
Kalaycı ÖA, Cömert FB, Hazer B, Atalay T, Cavicchi K, Çakmak M (2010) Synthesis, characterization, and antibacterial activity of metal nanoparticles embedded into amphiphilic comb-type graft copolymers. Polym Bull 65:215–226
Kim BJ, Bang J, Hawker CJ, Kramer EJ (2006) Effect chain density on the location of polymer-modified gold nanoparticles in a block copolymer template. Macromolecules 39:4108–4114
Kittel C (1986) Introduction to solid state physics. Wiley, New York
Kumbhakar P, Singh D, Tiwary CS, Mitra AK (2008) Chemical synthesis and visible photoluminescence emission from monodispersed ZnO nanoparticles. Chalcogenide Lett 5:387–394
Lemon BI, Crooks RM (2000) Preparation and characterization of dendrimer-encapsulated CdS semiconductor quantum dots. J Am Chem Soc 122:12886–12887
Liu SH, Qian XF, Yin J, Ma XD, Yuan JY, Zhu ZK (2003) Fabrication of CdS nanocrystals embedded in copolymer matrix by an in situ simultaneous copolymerization–sulfidation technique. J Phys Chem Solids 64:455–458
Mayer ABR (1998) Colloidal metal nanoparticles dispersed in amphiphilic polymers. Mater Sci Eng C 6:155
Mayer ABR (2001) Colloidal metal nanoparticles dispersed in amphiphilic polymers. Polym Adv Technol 12:96–106
Moffitt M, Vali H, Eisenberg A (1998) Spherical assemblies of semiconductor nanoparticles in water-soluble block copolymer aggregates. Chem Mater 10:1021–1028
Moore DE, Patel K (2001) Q-CdS photoluminescence activation on Zn+2 and Cd+2 salt introduction. Langmuir 17:2541–2544
Nabok A (2005) Organic inorganic nanostructures. Artech House Inc., Boston
Nicolais L, Carotenuto G (2005) Metal polymer nanocomposites. Wiley, Hoboken
Oren R, Liang Z, Barnard SJ, Warren SC, Wiesner U, Huck WTS (2009) Organization of nanoparticles in polymer brushes. J Am Chem Soc 131:1670–1671
Pandey S, Pandey AC (2009) Optical properties of hybrid composites based on highly luminescent CdS and ZnS nanocrystals in different polymer matrices. Transport and optical properties of nanomaterials. AIP Conf Proc 1147:216–222
Peretz S, Anghel DF, Theodor E, Stanciu G, Stoian C, Zgherea G, Florea-Spiroiu M (2011) Improving the properties of CdS nanoparticles by adding polymers. Part Sci Technol 29:229–241
Pesika NS, Stebe KJ, Searson PC (2003) Determination of the particle size distribution of quantum nanocrystals from absorbance spectra. Adv Mater 15:1289–1291
Petit C, Lixon P, Pileni MP (1990) Synthesis of cadmium sulfide in situ in reverse micelles. 2. Influence of the interface on the growth of the particles. J Phys Chem 94:1598–1603
Pomogailo AD (2000) Hybrid polymer–inorganic nanocomposites. Russ Chem Rev 69:53–80
Qi L, Collfen H, Antonietti M (2000) Synthesis and characterization of CdS nanoparticles stabilized by double-hydrophilic block copolymers. Nano Lett 1(2):61–65
Spanhel L, Haase M, Weller H, Henglein A (1987) Photochemistry of colloidal semiconductors. 20. Surface modification and stability of strong luminescing CdS particles. J Am Chem Soc 109:5649–5655
Talapin DV, Lee JS, Kovalenko MV, Shevchenko EV (2010) Prospects of colloidal nanocrystals for electronic and optoelectronic applications. Chem Rev 10:389–458
Tamborra M, Stricolli M, Comparelli R, Curri ML, Petrella A, Agostiano A (2004) Optical properties of hybrid composites based on highly luminescent CdS nanocrystals in polymer. Nanotechnology 15:240–244
Tang H, Yan M, Zhang H, Xia M, Yang D (2005) Preparation and characterization of water soluble CdS nanocrystals by surface modification of ethylene diamine. Mater Lett 59:1024–1027
Tessler N, Medvedev V, Kazes M, Kan SH, Banin U (2002) Efficient near-infrared polymer nanocrystal light-emitting diodes. Science 295:1506
Thakur P, Joshi SS, Kapoor S, Mukherjee T (2009) Fluorescence behavior of cysteine-mediated Ag@CdS nanocolloids. Langmuir 25:6377–6384
Tomczak N, Janczewski D, Han M, Vansco GJ (2009) Designer polymer-quantum dot architectures. Prog Polym Sci 34:393–430
Trindade T, O’Brien P, Pickett NL (2001) Nanocrystalline semiconductors: synthesis, properties and perspectives. Chem Mater 13:3843–3858
Tura C, Coombs N, Dag O (2005) One-pot synthesis of CdS nanoparticles in the channels of mesostructured silica films and monoliths. Chem Mater 17:573–579
Underhill RS, Liu G (2000) Triblock nanospheres and their use as templates for inorganic nanoparticle preparation. Chem Mater 12:2082
Wang J, Blau W (2009) Inorganic and hybrid nanostructures for optical limiting. Pure Appl Opt 11:024001
Yeh SW, Wei KH, Sun YS, Jeng US, Liang KS (2003) Morphological transformation of PS-b-PEO diblock copolymer by selectively dispersed colloidal CdS quantum dots. Macromolecules 36:7903–7907
Yıldız U, Hazer B, Tauer K (2012) Tailoring polymer architectures with macromonomer azo initiators. Polym Chem 3:1107–1118
Zhao H, Douglas EP (2002) Preparation of corona-embedded CdS nanoparticles. Chem Mater 14:1418–1423
Zhao H, Douglas EP, Benjamin SH, Schanze KS (2001) Preparation of CdS nanoparticles in salt induced block copolymer micelles. Langmuir 17:8428–8433
Acknowledgments
This work was supported by; both the Bulent Ecevit University Research Fund (#BEU-2012-10-03-13) and TUBITAK (Grant # 211T016).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kalaycı, Ö.A., Duygulu, Ö. & Hazer, B. Optical characterization of CdS nanoparticles embedded into the comb-type amphiphilic graft copolymer. J Nanopart Res 15, 1355 (2013). https://doi.org/10.1007/s11051-012-1355-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-012-1355-x