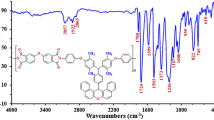
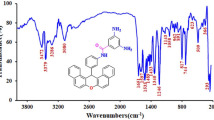
Abstract
A magnetic and fluorescent copolymer nanocomposite was prepared and characterized by various analytical techniques. Poly(epichlorohydrin) (PECH) was prepared by solution polymerization method using methacrylic acid as a chemical initiator and its copolymer was prepared by emulsion polymerization. After the structural modification of the copolymer with a nanohybrid system, its thermal properties were improved. The nanohybrid system consisting of Congo red dye decorated ferrite nanoparticles was synthesized by the conventional method and characterized by UV–Visible, fluorescence emission and excitation spectroscopy methods, high resolution transmission electron microscopy, and vibrating sample magnetometer techniques. Due to the decoration and encapsulation phenomena, the magnetic moment value of Fe3O4 was found to be decreased. While increasing the loading of nanohybrid system, the glass transition temperature (T g) of poly(methylmethacrylate) was increased. The TGA study inferred that by increasing the loading of nanohybrid system, the percentage weight residue remained above 550 °C was also increased. The chemical grafting of nanohybrid system onto the PECH-based copolymer backbone was confirmed by NMR spectroscopy and GPC analysis. The weight average molecular weight (M w) of the copolymer and nanohybrid-grafted copolymer exhibited higher M w than that of the PECH homopolymer. In the present study, we simply prepared a novel material and characterized it by various analytical methods.











Similar content being viewed by others
References
Knop K, Hoogenbeom R, Fischer D, Schubert US (2010) PEG in drug delivery: Pros and Cons as well as potential alternatives. Angew Chem Int Ed 49:6288–6308
Armand M (1990) Polymers with ionic conductivity. Adv Mater 2:278–286
Liu J, Sun J, Shen Z (1994) The polymerization of ECH with Nd(i-OPr)3–Al (i-Bu)3 system. Chin J Polym Sci 12:153–156
Nogueira AF, Spinace MAS, Gazotti WA, Girotto EM, de Paoli MA (2001) Poly(ethylene oxide-co-epichlorohydrin)/NaI: a promising polymer electrolyte for photoelectrochemical cells. Solid State Ionics 140:327–335
Bhosale SV, Rasool MA, Reina JA, Giamberini M (2013) New hybrid crystal columnar poly(epichlorohydrin-co-ethyleneoxide) derivatives leading biomimetic ion channels. Polym Eng Sci 53:159–167
Callau L, Reina JA, Manteaon A, Tessier M, Sparsky N (1999) Vinyl terminated side chain liquid crystalline derivatives containing biphenyl naphthalene mesogenic moieties. Macromolecules 32:7790–7797
Nouailhas H, Aouf C, Boutevin B, Fulcrand H, Guerneve CL, Caillol S (2011) Synthesis and preparation of bio based epoxy resins. Part 1. Glycidylation of flavonoids by epichlorohydrin. J Polym Sci Polym Chem 49:2261–2270
Alupei IC, Popa M, Abadie MJM, Hamcerencu M (2002) Super absorbent hydrogels based on xanthan and PVA—the study of the swelling properties. Eur Polym J 38:2313–2320
Han X, Shanks RA, Pavel D (2005) The synthesis and thermal properties of poly(epichlorohydrin) side chain liquid crystal polymers. Eur Polym J 41:984–991
Callau L, Reina JA, Mantecon A (2002) Cross linking of vinyl terminated side chain liquid crystalline polyethers using low molecular weight analogous mesogenic esters as reactive diluents. Polymer 43:6391–6396
Buruaga L, Pomposo JA (2011) Metal free PMMA nanoparticles by enamine click chemistry at room temperature. Polymers 3:1673–1683
Pathmamanoharan C, Slob C, Lekkerkerker HNW (1989) Preparation of poly(methylmethacrylate) lattices in non-polar media. Coilloid Polym Sci 267:448–450
Parzuchowski PG, Frost MC, Meyerhoff ME (2002) Synthesis and characterization based nitric oxide donors. J Am Chem Soc 124:12182–12191
Costache MC, Wang D, Heidecker MJ, Manias E, Wilkie CA (2006) The thermal degradation of PMMA nanocomposites with montmorillonite, layered double hydroxides and carbon nanotubes. Polym Adv Technol 17:272–280
Katsikas L, Avramovic M, Popovic IG, Cortes RDB, Milovanovic M, Kalagasidis-Krusic MT (2008) The thermal stability of PMMA by RAFT polymerisation. J Serb Chem Soc 73C:915–921
Bicak N, Gazi M, Tunca U, Kucukkaya I (2004) Utility of atom transfer radical polymerization for the preparation of PMMA beads in an aqueous suspension. J Polym Sci Polym Chem 42:1362–1366
Hammudi ZT, Nugay N, Nugay T (2004) Anionic polymerization of methyl methacrylate as promoted by a N-butyl lithium-pyridazine-polyether alkoxide based complex initiator system. Turk J Chem 28:387–394
Ahmad S, Ahmad S, Agnihotry SA (2007) Synthesis and characterization of in situ prepared PMMA nanocomposites. Bull Mater Sci 1:31–35
Nikolaidis AK, Achilias DS, Karayannidis GP (2011) Synthesis and characterization of PMMA/organo modified montmorillonite nanocomposites prepared by in situ bulk polymerization. Ind Eng Chem Res 50:571–579
Klein SM, Manoharan VN, Pine DJ, Lange FF (2003) Preparation of monodisperse PMMA microsphere in non-polar solvents by dispersion polymerisation with a macromonomeric stabilizer. Colloid Polym Sci 282:7–13
Gong S, Ma H, Wan X (2006) Atom transfer radical polymerization of methyl methacrylate induced by an initiator derived from an ionic liquid. Polym Int 55:1420–1425
Tonga JD, Leclere PH, Doneux C, Bredas JL, Lazzaroni R, Jerome R (2000) Synthesis and bulk properties of poly(methyl methacrylate)-b-poly(isooctyl acrylate)-b-poly(methyl methacrylate). Polymer 41:4617–4624
Nájera MA, Elizalde LE, Vázquez Y, Gdl Santos (2009) Synthesis of random copolymers poly(methylmethacrylate-co-azo monomer) by ATRP-AGET. Macromol Symp 283–284:51–55
Tang T, Castelletto V, Parras P, Hamley IW, King SM, Roy D, Perrier S, Hoogenboom R, Schubert US (2006) Thermo-responsive poly(methyl methacrylate)-block-poly(N-isopropylacrylamide) block copolymers synthesized by RAFT polymerization micellization and gelation. Macromol Chem Phys 207:1718–1726
Król P, Chmielarz P (2013) Synthesis of PMMA-b-PU-b-PMMA tri-block copolymers through ARGET ATRP in the presence of air. Express Polym Lett 7:249–260
Gultek A (2010) Synthesis and characterization of hybrid congo red from chloro-functionalized silsesquioxanes. Turk J Chem 34:437–445
Dafare S, Deshpande PS, Bhavsar RS (2013) Photocatalytic degradation of congo red dye on combustion synthesised Fe2O3. Ind J Chem Technol 20:406–410
Movahedi M, Mahjoub AR, Janitabar S (2009) Photodegradation of congo red in aqueous solution on ZnO as an alternative catalyst to TiO2. J Iran Chem Soc 6:570–577
Hu S, Yan P, Maslov K, Lee JM, Wang LV (2009) Intra vital imaging of amyloid plaques in a transgenic mouse model using optical resolution photoacoustic micros. Opt Lett 34:3899–3901
Mathur T, Singhal S, Khan S, Upadhyay DJ, Fatma T, Rattan A (2006) Detection of biofilm formation among the clinical isolates of staphylococci: an evaluation of three different screening methods. Indian J Med Microbiol 24:25–29
Kumar P, Selvi SS, Govindaraju M (2012) In vitro anti-biofilm and anti-bacterial activity of Junceella juncea for its biomedical application. Asian Pac J Trop Biomed 2:930–935
Li X, Qian Y, Liu T, Hu X, Zhang G, You Y, Liu S (2011) Amphiphilic multiarm star block copolymer-based multifunctional unimolecular micelles for cancer targeted drug delivery and MR imaging. Biomaterials 32:6595–6605
Meenarathi B, Palanikumar S, Kannammal L, Anbarasan R (2015) Synthesis, characterization and catalytic activity of Ag-acidfuchsin nanohybrid system towards the ring opening polymerization of ε-caprolactone. Spectrochim Acta A Mol Biomol Spectrosc 135:93–100
Murugesan A, Meenarathi B, Palanikumar S, Kannammal L, Anbarasan R (2014) Synthesis, characterization and drug delivery activity of poly(anthranilic acid) based triblock copolymer. Synth Met 189:143–151
Bernogozzi I, Torrengo S, Minati L, Ferrari M, Chiappini A, Armellini C, Toniutti L, Lunelli L, Speranza G (2012) Synthesis and characterization of PMMA-based superhydrophobic surfaces. Colloid Polym Sci 290:315–322
Fang FF, Kim JH, Choi HJ, Kim CA (2009) Synthesis and electro rheological response of nanosized laponite stabilized PMMA. Colloid Polym Sci 287:745–749
Meenarathi B, Kannammal L, Palanikumar S, Anbarasan R (2014) Synthesis and characterization of Fe3O4-acidfuchsin tagged poly(ε-caprolactone) nanocomposites. Mater Res Exp 1:1–16
Meenarathi Chen HH, Chen PH, Anbarasan R (2013) NIR dye functionalized dye MWCNT as an effective initiator for the ring opening polymerization of ε-Caprolactone. J Polym Res 20:118–130
Palanikumar S, Siva P, Meenarathi B, Kannammal L, Anbarasan R (2014) Effect of Fe3O4 on the sedimentation and structure-property relationship of starch under different pHs. Int J Biol Macromol 67:91–98
Zhao Q, Samulski ET (2006) A comparative study of PMMA and poly(styrene)/clay nanocomposites prepared in super critical CO2. Polymer 47:663–671
Aldosari MA, Othman AA, Alsharaeh EH (2013) Synthesis and characterization of the in situ bulk polymerization of poly(methylmethacrylate) containing graphene sheets using microwave irradiation. Molecules 18:3152–3167
Zhang B, Blum FD (2002) Thermogravimetric studies of poly(methylmethacrylate) on silica. Polym Prepr 43:484–485
Aouak T, Deraz NM, Alarifi AS (2013) Synthesis, non-isothermal crystallization and magnetic properties of Co0.25Zn0.25Fe2O4/poly(ethylene-co-vinylalcohol) nanocomposite. Bull Mater Sci 36:417–427
Singh JP, Dixit G, Negi P, Sirvastava RC, Negi P, Agrawal HM, Kumar R (2013) HRTEM and FTIR investigation of nanosized Zinc ferrite irradiated with 100 MeV oxygen ions. Spectrochim Acta Part A 107:326–333
Mahdavinia GR, Iravani S, Zoroufi S, Hosseinzadeh H (2014) Magnetic and K+ cross linked kappa carregeenan nanocomposite beads and adsorption of crystal violet. Iran Polym J 23:335–344
Acknowledgments
We sincerely acknowledged Mrs. G. Vijayalakshmi, Assistant Professor of English Department for her valuable help during the manuscript preparation.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
13726_2015_354_MOESM1_ESM.tif
Fig. S1 UV–Visible absorbance (a) fluorescence emission (b), and fluorescence excitation (c) spectra of CR dye (TIFF 126 kb)
13726_2015_354_MOESM2_ESM.tif
Fig. S2 UV–Visible absorbance (a) fluorescence emission (b), and fluorescence excitation (c) spectra of Fe3O4/CR nanohybrid system (TIFF 137 kb)
13726_2015_354_MOESM3_ESM.tif
Fig. S3 VSM values of (a) Fe3O4, (b) Fe3O4/CR nanohybrid system and (c) MA-PECH-PMMA-Fe3O4/CR (0.3 g) nanocomposite sample (TIFF 155 kb)
Rights and permissions
About this article
Cite this article
Luna Eunice, S., Meenarathi, B., Palanikumar, S. et al. Synthesis and characterisation of poly(epichlorohydrin-g-Fe3O4/congo red)-co-poly(methylmethacrylate). Iran Polym J 24, 651–661 (2015). https://doi.org/10.1007/s13726-015-0354-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13726-015-0354-z