Abstract
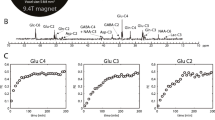
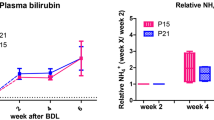
Chronic liver disease (CLD) leads to a spectrum of neuropsychiatric disorders named hepatic encephalopathy (HE). Even though brain energy metabolism is believed to be altered in chronic HE, few studies have explored energy metabolism in CLD-induced HE, and their findings were inconsistent. The aim of this study was to characterize for the first time in vivo and longitudinally brain metabolic changes in a rat model of CLD-induced HE with a focus on energy metabolism, using the methodological advantages of high field proton and phosphorus Magnetic Resonance Spectroscopy (1H- and 31P-MRS). Wistar rats were bile duct ligated (BDL) and studied before BDL and at post-operative weeks 4 and 8. Glutamine increased linearly over time (+146 %) together with plasma ammonium (+159 %). As a compensatory effect, other brain osmolytes decreased: myo-inositol (-36 %), followed by total choline and creatine. A decrease in the neurotransmitters glutamate (-17 %) and aspartate (-28 %) was measured only at week 8, while no significant changes were observed for lactate and phosphocreatine. Among the other energy metabolites measured by 31P-MRS, we observed a non-significant decrease in ATP together with a significant decrease in ADP (-28 %), but only at week 8 after ligation. Finally, brain glutamine showed the strongest correlations with changes in other brain metabolites, indicating its importance in type C HE. In conclusion, mild alterations in some metabolites involved in energy metabolism were observed but only at the end stage of the disease when edema and neurological changes are already present. Therefore, our data indicate that impaired energy metabolism is not one of the major causes of early HE symptoms in the established model of type C HE.




Similar content being viewed by others
References
Barbiroli B, Gaiani S, Lodi R et al (2002) Abnormal brain energy metabolism shown by in vivo phosphorus magnetic resonance spectroscopy in patients with chronic liver disease. Brain Res Bull 59:75–82. doi:10.1016/S0361-9230(02)00839-0
Biecker E, De Gottardi A, Neef M et al (2005) Long-term treatment of bile duct-ligated rats with rapamycin (sirolimus) significantly attenuates liver fibrosis: analysis of the underlying mechanisms. J Pharmacol Exp Ther 313:952–961. doi:10.1124/jpet.104.079616
Blei AT, Córdoba J (2001) Hepatic encephalopathy. Am J Gastroenterol 96:1968–1976. doi:10.1111/j.1572-0241.2001.03964.x
Bluml S, Zuckerman E, Tan J, Ross BD (1998) Proton-decoupled 31P magnetic resonance spectroscopy reveals osmotic and metabolic disturbances in human hepatic encephalopathy. J Neurochem 71:1564–1576
Bosoi CR, Parent-Robitaille C, Anderson K et al (2011) AST-120 (spherical carbon adsorbent) lowers ammonia levels and attenuates brain edema in bile duct-ligated rats. Hepatology 53:1995–2002. doi:10.1002/hep.24273
Bosoi CR, Zwingmann C, Marin H et al (2014) Increased brain lactate is central to the development of brain edema in rats with chronic liver disease. J Hepatol 60:554–560. doi:10.1016/j.jhep.2013.10.011
Bothwell JH, Styles P, Bhakoo KK (2002) Swelling-activated taurine and creatine effluxes from rat cortical astrocytes are pharmacologically distinct. J Membr Biol 185:157–164. doi:10.1007/s00232-001-0121-2
Braissant O (2010) Ammonia toxicity to the brain: effects on creatine metabolism and transport and protective roles of creatine. Mol Genet Metab 100(Suppl):S53–8. doi:10.1016/j.ymgme.2010.02.011
Braissant O, Henry H, Villard A-M et al (2002) Ammonium-induced impairment of axonal growth is prevented through glial creatine. J Neurosci 22:9810–9820
Braissant O, McLin VA, Cudalbu C (2013) Ammonia toxicity to the brain. J Inherit Metab Dis 36:595–612. doi:10.1007/s10545-012-9546-2
Brusilow SW, Traystman R (1986) Hepatic encephalopathy. N Engl J Med 314:786–787, author reply 787
Brusilow SW, Koehler RC, Traystman RJ, Cooper AJL (2010) Astrocyte glutamine synthetase: importance in hyperammonemic syndromes and potential target for therapy. Neurotherapeutics 7:452–470. doi:10.1016/j.nurt.2010.05.015
Butterworth RF (2003) Pathogenesis of hepatic encephalopathy: new insights from neuroimaging and molecular studies. J Hepatol 39:278–285. doi:10.1016/S0168-8278(03)00267-8
Butterworth RF, Norenberg MD, Felipo V et al (2009) Experimental models of hepatic encephalopathy: ISHEN guidelines. Liver Int 29:783–788. doi:10.1111/j.1478-3231.2009.02034.x
Chavarria L, Oria M, Romero-Gimenez J et al (2010) Diffusion tensor imaging supports the cytotoxic origin of brain edema in a rat model of acute liver failure. Gastroenterology 138:1566–1573. doi:10.1053/j.gastro.2009.10.003
Chavarria L, Oria M, Romero-Giménez J et al (2013) Brain magnetic resonance in experimental acute-on-chronic liver failure. Liver Int 33:294–300. doi:10.1111/liv.12032
Cooper JLA, Plum F (1987) Biochemistry and physiology of brain ammonia. Physiol Rev 67:440–519
Córdoba J, Alonso J, Rovira A et al (2001) The development of low-grade cerebral edema in cirrhosis is supported by the evolution of (1)H-magnetic resonance abnormalities after liver transplantation. J Hepatol 35:598–604
Cudalbu C (2013) In vivo studies of brain metabolism in animal models of Hepatic Encephalopathy using 1H Magnetic Resonance Spectroscopy. Metab Brain Dis 28:167–174. doi:10.1007/s11011-012-9368-9
Cudalbu C, Mlynárik V, Gruetter R (2012) Handling macromolecule signals in the quantification of the neurochemical profile. J Alzheimers Dis 31(Suppl 3):S101–S115. doi:10.3233/JAD-2012-120100
Cudalbu C, Rackayova V, McLin VA, Braissant O (2014) Brain Glutamine, Osmolytes and Edema in a Model of Chronic Hepatic Encephalopathy: in vivo and Londitudinal Measurements using 1H MRS, DTI and Immunofluorescence. 16th ISHEN Meet London. doi: 10.1002/yea.122
Dam G, Keiding S, Munk OL et al (2013) Hepatic encephalopathy is associated with decreased cerebral oxygen metabolism and blood flow, not increased ammonia uptake. Hepatology 57:258–265. doi:10.1002/hep.25995
Davies NA, Wright G, Ytrebø LM et al (2009) L-ornithine and phenylacetate synergistically produce sustained reduction in ammonia and brain water in cirrhotic rats. Hepatology 50:155–164. doi:10.1002/hep.22897
Felipo V, Butterworth RF (2002) Neurobiology of ammonia. Prog Neurobiol 67:259–279
Ferenci P, Lockwood A, Mullen K et al (2002) Hepatic encephalopathy--definition, nomenclature, diagnosis, and quantification: final report of the working party at the 11th World Congresses of Gastroenterology, Vienna, 1998. Hepatology 35:716–721. doi:10.1053/jhep.2002.31250
Flögel U, Niendorf T, Serkowa N et al (1995) Changes in organic solutes, volume, energy state, and metabolism associated with osmotic stress in a glial cell line: a multinuclear NMR study. Neurochem Res 20:793–802
Golding EM, Teague WE, Dobson GP (1995) Adjustment of K’ to varying pH and pMg for the creatine kinase, adenylate kinase and ATP hydrolysis equilibria permitting quantitative bioenergetic assessment. J Exp Biol 198:1775–1782
Gruetter R, Tkác I (2000) Field mapping without reference scan using asymmetric echo-planar techniques. Magn Reson Med 43:319–323
Häussinger D (2006) Low grade cerebral edema and the pathogenesis of hepatic encephalopathy in cirrhosis. Hepatology 43:1187–1190. doi:10.1002/hep.21235
Häussinger D, Kircheis G, Fischer R et al (2000) Hepatic encephalopathy in chronic liver disease: a clinical manifestation of astrocyte swelling and low-grade cerebral edema? J Hepatol 32:1035–1038
Hazell AS, Butterworth RF (1999) Hepatic encephalopathy: an update of pathophysiologic mechanisms. Proc Soc Exp Biol Med 222:99–112
Iotti S, Frassineti C, Alderighi L et al (1996) In vivo assessment of free magnesium concentration in human brain by 31P MRS. A new calibration curve based on a mathematical algorithm. NMR Biomed 9:24–32. doi:10.1002/(SICI)1099-1492(199602)9:1<24::AID-NBM392>3.0.CO;2-B
Kale RA, Gupta RK, Saraswat VA et al (2006) Demonstration of interstitial cerebral edema with diffusion tensor MR imaging in type C hepatic encephalopathy. Hepatology 43:698–706. doi:10.1002/hep.21114
Kosenko E, Kaminsky Y, Grau E et al (1994) Brain ATP depletion induced by acute ammonia intoxication in rats is mediated by activation of the NMDA receptor and Na+, K+ ATPase. J Neurochem 63:2172–2178
Kreis R, Farrow N, Ross BD (1991) Localized 1H NMR spectroscopy in patients with chronic hepatic encephalopathy. Analysis of changes in cerebral glutamine, choline and inositols. NMR Biomed 4:109–116
Kreis R, Ross BD, Farrow NA, Ackerman Z (1992) Metabolic disorders of the brain in chronic hepatic encephalopathy detected with H-1 MR spectroscopy. Radiology 182:19–27. doi:10.1148/radiology.182.1.1345760
Kuby SA, Noda L, Lardy HA (1954) Adenosinetriphosphate-creatine transphosphorylase. J Biol Chem 210:65–82
Lai JC, Cooper AJ (1991) Neurotoxicity of ammonia and fatty-acids - differential inhibition of mitochondrial dehydrogenases by ammonia and fatty acyl coenzyme a derivatives. Neurochem Res 16:795–803
Lanz B, Cudalbu C, Mclin V, et al. (2014) Effects of chronic hepatic encephalopathy on brain energy metabolism , studied by in vivo 13 C MRS in rats. 16th ISHEN Meet. London. pp 1–2
Lavoie J, Giguère JF, Layrargues GP, Butterworth RF (1987) Amino acid changes in autopsied brain tissue from cirrhotic patients with hepatic encephalopathy. J Neurochem 49:692–697
Leke R, Bak LK, Iversen P et al (2011) Synthesis of neurotransmitter GABA via the neuronal tricarboxylic acid cycle is elevated in rats with liver cirrhosis consistent with a high GABAergic tone in chronic hepatic encephalopathy. J Neurochem 117:824–832. doi:10.1111/j.1471-4159.2011.07244.x
Leke R, de Oliveira DL, Mussulini BHM et al (2012) Impairment of the organization of locomotor and exploratory behaviors in bile duct-ligated rats. PLoS One 7, e36322. doi:10.1371/journal.pone.0036322
Lien YHH, Shapiro JI, Chan L (1990) Effects of hypernatremia on organic brain osmoles. J Clin Invest 85:1427–1435. doi:10.1172/JCI114587
Lockwood AH, Ginsberg MD, Rhoades HM, Gutierrez MT (1986) Cerebral glucose metabolism after portacaval shunting in the rat. Patterns of metabolism and implications for the pathogenesis of hepatic encephalopathy. J Clin Invest 78:86–95. doi:10.1172/JCI112578
McPhail MJW, Patel NR, Taylor-Robinson SD (2012) Brain Imaging and hepatic encephalopathy. Clin Liver Dis 16:57–72. doi:10.1016/j.cld.2011.12.001
Mlynárik V, Gambarota G, Frenkel H, Gruetter R (2006) Localized short-echo-time proton MR spectroscopy with full signal-intensity acquisition. Magn Reson Med 56:965–970. doi:10.1002/mrm.21043
Mlynárik V, Cacquevel M, Sun-Reimer L et al (2012) Proton and phosphorus magnetic resonance spectroscopy of a mouse model of Alzheimer’s disease. J Alzheimers Dis 31(Suppl 3):S87–S99. doi:10.3233/JAD-2012-112072
Nioka S, Chance B, Hilberman M et al (1987) Relationship between intracellular pH and energy metabolism in dog brain as measured by 31P-NMR. J Appl Physiol 62:2094–2102
Ott P, Vilstrup H (2014) Cerebral effects of ammonia in liver disease: current hypotheses. Metab Brain Dis 29:901–911. doi:10.1007/s11011-014-9494-7
Ott P, Clemmesen O, Larsen FS (2005) Cerebral metabolic disturbances in the brain during acute liver failure: From hyperammonemia to energy failure and proteolysis. Neurochem Int 47:13–18. doi:10.1016/j.neuint.2005.04.002
Petroff O, Prichard J (1985) Cerebral intracellular pH by 31P nuclear magnetic resonance. Neurology 35:781–788
Provencher SW (2001) Automatic quantitation of localized in vivo 1H spectra with LCModel. NMR Biomed 14:260–264. doi:10.1002/nbm.698
Quinn M, McMillin M, Galindo C et al (2014) Bile acids permeabilize the blood brain barrier after bile duct ligation in rats via Rac1-dependent mechanisms. Dig Liver Dis 46:527–534. doi:10.1016/j.dld.2014.01.159
Rackayova V, Lanz B, Berset C, et al. (2015) In vivo Longitudinal Measurements of Brain Energy Metabolism in Chronic Hepatic Encephalopathy in a Rat Model using 31P MRS and 1H MRS. 23rd Annu. ISMRM Meet. Toronto. p 1
Rae CD (2014) A guide to the metabolic pathways and function of metabolites observed in human brain 1H magnetic resonance spectra. Neurochem Res 39:1–36. doi:10.1007/s11064-013-1199-5
Rama Rao KV, Norenberg MD (2012) Brain energy metabolism and mitochondrial dysfunction in acute and chronic hepatic encephalopathy. Neurochem Int 60:697–706. doi:10.1016/j.neuint.2011.09.007
Rao KVR, Norenberg MD (2001) Cerebral energy metabolism in hepatic encephalopathy and hyperammonemia. Metab Brain Dis 16:67–78. doi:10.1023/A:1011666612822
Ratnakumari L, Audet R, Qureshi IA, Butterworth RF (1995) Na+, K+−ATPase activities are increased in brain in both congenital and acquired hyperammonemic syndromes. Neurosci Lett 197:89–92
Schousboe A, Waagepetersen HS, Leke R, Bak LK (2014) Effects of hyperammonemia on brain energy metabolism: controversial findings in vivo and in vitro. Metab Brain Dis. doi:10.1007/s11011-014-9513-8
Shah NJ, Neeb H, Kircheis G et al (2008) Quantitative cerebral water content mapping in hepatic encephalopathy. Neuroimage 41:706–717. doi:10.1016/j.neuroimage.2008.02.057
Taylor-Robinson SD, Sargentoni J, Oatridge A et al (1996) MR imaging and spectroscopy of the basal ganglia in chronic liver disease: correlation of T1-weighted contrast measurements with abnormalities in proton and phosphorus-31 MR spectra. Metab Brain Dis 11:249–268
Taylor-Robinson SD, Buckley C, Changani KK et al (1999) Cerebral proton and phosphorus-31 magnetic resonance spectroscopy in patients with subclinical hepatic encephalopathy. Liver 19:389–398. doi:10.1111/j.1478-3231.1999.tb00067.x
Tkáč I, Starčuk Z, Choi IY, Gruetter R (1999) In vivo 1H NMR spectroscopy of rat brain at 1 ms echo time. Magn Reson Med 41:649–656. doi:10.1002/(SICI)1522-2594(199904)41:4<649::AID-MRM2>3.0.CO;2-G
Tkac I, Henry P-G, Zacharoff L et al (2012) Homeostatic adaptations in brain energy metabolism in mouse models of Huntington disease. J Cereb Blood Flow Metab 32:1977–1988. doi:10.1038/jcbfm.2012.104
Vanhamme L, van den Boogaart A, Van Huffel S (1997) Improved method for accurate and efficient quantification of MRS data with use of prior knowledge. J Magn Reson 129:35–43
Veech RL, Lawson JW, Cornell NW, Krebs HA (1979) Cytosolic phosphorylation potential. J Biol Chem 254:6538–6547
Zwingmann C (2007) The anaplerotic flux and ammonia detoxification in hepatic encephalopathy. Metab Brain Dis 22:235–249. doi:10.1007/s11011-007-9069-y
Zwingmann C, Butterworth R (2005) An update on the role of brain glutamine synthesis and its relation to cell-specific energy metabolism in the hyperammonemic brain: further studies using NMR spectroscopy. Neurochem Int 47:19–30. doi:10.1016/j.neuint.2005.04.003
Acknowledgments
Supported by Centre d’Imagerie BioMédicale (CIBM) of the UNIL, UNIGE, HUG, CHUV, EPFL, the Leenaards and Jeantet Foundations. EU Grant FP7-PEOPLE-2012-ITN project 316679 TRANSACT. The authors thank Prof. Rolf Gruetter (Centre d’Imagerie BioMédicale (CIBM), École Polytechnique Fédérale de Lausanne (EPFL), Lausanne, Switzerland) for his support and Dr Vladimír Mlynárik (Centre d’Imagerie BioMédicale (CIBM), École Polytechnique Fédérale de Lausanne (EPFL), Lausanne, Switzerland) for his advices on 31P-MRS.
Conflict of Interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
Calculations of energy metabolism parameters
Intracellular pH was calculated based on the difference in resonance frequencies between Pi and PCr signals (δPi) in ppm (parts per million) using the Henderson-Hasselbalch equation
(Petroff and Prichard 1985).
Similarly, [Mg2+] was assessed from the difference in resonance frequencies between β-ATP and PCr signals (δβ-ATP) in ppm using equation
(Iotti et al. 1996).
ADP concentration was calculated as
with an apparent equilibrium constant of biochemical creatine kinase reaction (KCK) (where the reactant concentrations are the sum of all the ionic and metal complex species at specified pH and [Mg2+]) adapted for each particular pH and [Mg2+] of each individual rat as described previously (Golding et al. 1995). [ATP], [Cr] and [PCr] were measured by 1H- and 31P-MRS, pH and [Mg2+] from Eqs. (1) and (2) respectively.
The phosphorylation potential (PP) representing the immediately available high energy phosphate pool was calculated as
(Veech et al. 1979).
The percentage of the maximal rate of ATP biosynthesis (v/Vmax-ATP) was calculated according to
(Veech et al. 1979; Nioka et al. 1987).
Finally the relative rate of CK reaction (v/Vmax-CK) was determined from
(Veech et al. 1979), with Km- ADP = 0.8 mmol/l and Km-PCr = 5.0 mmol/l (Kuby et al. 1954).
Rights and permissions
About this article
Cite this article
Rackayova, V., Braissant, O., McLin, V.A. et al. 1H and 31P magnetic resonance spectroscopy in a rat model of chronic hepatic encephalopathy: in vivo longitudinal measurements of brain energy metabolism. Metab Brain Dis 31, 1303–1314 (2016). https://doi.org/10.1007/s11011-015-9715-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-015-9715-8