Abstract
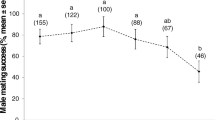
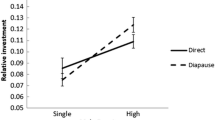
Male investment in spermatophore quality is a function of the degree of polyandry. Among butterflies and other organisms that provide gifts, polyandry tends to select for gifts of better quality than monandry. Tropical butterfly species Heliconius erato and H. melpomene differ in mating systems and provide an ideal system to test expectations regarding allocation to spermatophore quality and production. Heliconius erato males mate with females while they emerge from pupa. Females mate for life and show no mate choice. In H. melpomene, females are courted by males and may mate several times in their lifetime. In order to evaluate whether spermatophore quality (mass and protein content) differed between the two species we conducted mating experiments with virgin males and virgin females in an outdoor insectary. In order to study the dynamics of spermatophore production during subsequent matings, males were also allowed to mate two or more times. Spermatophores of virgin H. melpomene males contained significantly more protein than those of H. erato, confirming theoretical expectations; spermatophore mass was not different. The polyandrous species also showed a significant reduction in mass and protein content in the second mating, while H. erato responses were mixed but non significant. Additionally, the effect of time between successive matings was dependent on species identity. Our results confirm that polyandrous Heliconius invest more in spermatophore quality than its monandrous congener. Two explanations are suggested for the observed loss of mass and protein content in polyandrous Heliconius, potential cost for production or strategic ejaculation.




Similar content being viewed by others
References
Arnqvist G, Nilsson T (2000) The evolution of polyandry: multiple mating and female fitness in insects. Anim Behav 60:145–164
Beltrán M, Jiggins CD, Brower AVZ, Bermingham E, Mallet J (2007) Do pollen feeding, pupal-mating and larval gregariousness have a single origin in Heliconius butterflies? Inferences from multilocus DNA sequence data. Biol J Linn Soc 92:221–239
Bissoondath CJ, Wiklund C (1995) Protein content of spermatophores in relation to monandry/polyandry in butterflies. Behav Ecol Sociobiol 37:365–371
Bissoondath CJ, Wiklund C (1996) Male butterfly investment in successive ejaculates in relation to mating system. Behav Ecol Sociobiol 39:285–292
Boggs CL (1981a) Selection pressures affecting male nutrient investment at mating in heliconiine butterflies. Evolution 35:931–940
Boggs CL (1981b) Nutritional and life-history determinants of resource allocation in holometabolous insects. Am Nat 117:692–709
Boggs CL (1990) A general model of the role of male-donated nutrients in female insects' reproduction. Am Nat 136:598–617
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Caballero-Mendieta N, Cordero C (2013) Male mating costs in a butterfly that produces small ejaculates. Physiol Entomol 38:318–325
Cardoso MZ, Gilbert LE (2007) A male gift to its partner? Cyanogenic glycosides in the spermatophore of longwing butterflies (Heliconius). Naturwissenschaften 94:39–42
Cardoso MZ, Roper JJ, Gilbert LE (2009) Prenuptial agreements: mating frequency predicts gift-giving in Heliconius species. Entomologia Experimentalis et Applicata 131:109–114
Cratsley CK, Rooney JA, Lewis SM (2003) Limits to nuptial gift production by male fireflies, Photinus ignitus. J Insect Behav 16:361–370
Deinert EI (2003) Mate location and competition for mates in a pupal mating butterfly. In: Boggs CL, Watt WB, Ehrlich PR (eds) Butterflies: ecology and evolution taking flight. Chicago University Press, Chicago, pp. 91–108
Dunlap-Pianka H (1979) Ovarian dynamics in Heliconius butterflies: correlations among daily oviposition rates, egg weights, and quantitative aspects of oogenesis. J Insect Physiol 25:741–749
Ehrlich PR, Gilbert LE (1973) Population structure and dynamics of the tropical butterfly Heliconius ethilla. Biotropica 5:69–82
Gage MJG (1991) Risk of sperm competition directly affects ejaculate size in the Mediterranean fruit fly. Anim Behav 42:1036–1037
Gilbert LE (1972) Pollen feeding and reproductive biology of Heliconius butterflies. Proc Natl Acad Sci 69:1403–1407
Gilbert LE (1991) Biodiversity of a Central American Heliconius community: pattern, process, and problems. In: Price P, Lewinsohn TM, Fernandes GW, Benson WW (eds) Plant-animal interactions: evolutionary ecology in tropical and temperate regions. Wiley, New York, pp. 403–427
Gilbert LE (2003) Adaptive novelty through introgression in Heliconius wing patterns: evidence for shared genetic “tool box” from synthethic hybrid zones and a theory of diversification . In: Boggs CL, Watt WB, Ehrlich PR (eds) Ecology and evolution taking flight: butterflies as model systems. University of Chicago Press, Chicago, pp. 281–318
Gwynne DT (2008) Sexual conflict over nuptial gifts in insects. Annu Rev Entomol 53:83–101
Hughes L, Chang BS-W, Wagner D, Pierce NE (2000) Effects of mating history on ejaculate size, fecundity, longevity, and copulation duration in the ant-tended lycaenid butterfly, Jalmenus evagoras. Behav Ecol Sociobiol 47:119–128
Kelly CD, Jennions MD (2011) Sexual selection and sperm quantity: meta-analyses of strategic ejaculation. Biol Rev 86:863–884
Klein AL, Araújo AM (2010) Courtship behavior of Heliconius erato phyllis (Lepidoptera: Nymphalidae) towards virgin and mated females: conflict between attraction and repulsion signals? J Ethol 28:409–420
Lederhouse RC, Ayres MP, Scriber JM (1990) Adult nutrition affects males virility in Papilio glaucus L. Funct Ecol 4:743–751
Merril RM, Dasmahapatra KK, Davey JW, Dell'Aglio DD, Hanly JJ, Huber B, Jiggins CD, Joron M, Kozak KM, Llaurens V, Martin SH, Montgomery SH, Morris J, Nadeau NJ, Pinharanda AL, Rosser N, Thompson MJ, Vanjari S, Wallbank RWR, Yu Q (2015) The diversification of Heliconius butterflies: what have we learned in 150 years? J Evol Biol 28:1317–1438
Perry JC, Rowe L (2010) Condition-dependent ejaculate size and composition in a ladybird beetle. Proc R Soc B Biol Sci 277:3639–3647
R Core Team (2015) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL http://www.R-project.org/
Schultz S, Estrada C, Yildizhan S, Boppré M, Gilbert LE (2008) An antiaphrodisiac in Heliconius melpomene butterflies. J Chem Ecol 34:82–93
Svärd L, Wiklund C (1986) Different ejaculate delivery strategies in first versus subsequent matings in the swallowtail butterfly Papilio machaon L. Behav Ecol Sociobiol 18:325–330
Svärd L, Wiklund C (1989) Mass and production rate of ejaculates in relation to monandry/polyandry in butterflies. Behav Ecol Sociobiol 24:395–402
Takeuchi T (2012) Cost of reproduction in males of a satyrine butterfly Lethe diana. Physiol Entomol 37:171–176
Vahed K (1998) The function of nuptial feeding in insects: a review of empirical studies. Biol Rev 73:43–78
Walters JR, Harrison RG (2010) Combined EST and proteomic analysis identifies rapidly evolving seminal fluid proteins in Heliconius butterflies. Mol Biol Evol 27:2000–2013
Walters JR, Harrison RG (2011) Decoupling of rapid and adaptive evolution among seminal fluid proteins in Heliconius butterflies with divergent mating systems. Evolution 65:2855–2871
Wiklund C (2003) Sexual selection and the evolution of butterfly mating systems. In: Boggs CL, Watt WB, Ehrlich PR (eds) Butterflies: ecology and evolution taking flight. Chicago University Press, Chicago, pp. 67–90
Wiklund C, Karlsson B, Leimar O (2001) Sexual conflict and cooperation in butterfly reproduction: a comparative study of polyandry and female fitness. Proc R Soc B Biol Sci 268:1661–1667
Wiklund C, Gotthard K, Nylin S (2003) Mating system and the evolution of sex-specific mortality rates in two nymphalid butterflies. Proc R Soc B Biol Sci 270:1823–1828
Zagrobelny M, Bak S, Olsen CE, Møller BL (2007) Intimate roles for cyanogenic glucosides in the life cycle of Zygaena filipendulae (Lepidoptera, zygaenidae). Insect Biochem Mol Biol 37:1189–1197
Zagrobelny M, Bak S, Møller BL (2008) Cyanogenesis in plants and arthropods. Phytochemistry 69:1457–1468
Zagrobelny M, Motawia MS, Olsen CE, Bak S, Møller BL (2013) Male-to-female transfer of 5-hydroxytryptohan glucoside during mating in Zygaena filipendula (Lepidoptera). Insect Biochem Mol Biol 43:1037–1044
Acknowledgments
We thank Mauricio Sales (Department of Biochemistry, UFRN, in memoriam) for helping with protein analysis. We also thank Carlos Fonseca and Eduardo Venticinque for discussions regarding data analysis, and Paulo Enrique Peixoto and three anonymous reviewers for commenting on the manuscript. MZC thanks CNPq for a research productivity grant (Proc. 306863/2010–3).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cardoso, M.Z., Silva, E.S. Spermatophore Quality and Production in two Heliconius Butterflies with Contrasting Mating Systems. J Insect Behav 28, 693–703 (2015). https://doi.org/10.1007/s10905-015-9536-y
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10905-015-9536-y