Summary
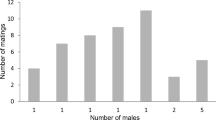
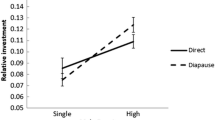
The mating system maintained in a species has a strong effect on the degree of sperm competition, and certainty of paternity should accordingly influence the optimal sperm content, nutrient content, and mass of the ejaculate. We investigated how ejaculate mass relates to the degree of polyandry in 20 species of butterflies belonging to the families Pieridae and Satyridae. We found that the degree of polyandry has a substantial effect on the reproductive performance of males. The allometric line between ejaculate mass and male body mass has a higher elevation in the pierids compared to the satyrids. The mean number of matings performed by the pierid species is also higher compared to the mean of the satyrids. Thus, the relative ejaculate mass is larger in the family in which polyandry is more pronounced. A within family effect of degree of polyandry on relative ejaculate mass was also detected in the pierids. Since males of polyandrous species on average mate more often than males of monandrous species, they should be expected to have a higher capacity for producing many ejaculates. We investigated how this capacity was influenced by the degree of polyandry, by allowing males of seven different species (Danaus plexippus, Lasiommata megera, Papilio machaon, Pararge aegeria, Pieris napi, Pieris rapae, and Polygonia c-album) to mate twice, with different time intervals between matings. The results showed that not only is the mass of the ejaculate greater in more polyandrous species, but also the rate at which males are able to produce sperm and accessory substances is greater. Hence our data indicate that sperm competition is important for explaining variation in ejaculate mass in butterflies.
Similar content being viewed by others
References
Benz G (1969) Influence of mating, insemination and other factors on oogenesis and oviposition in the moth Zeraphera diniana. J Insect Physiol 15:55–71
Boggs CL (1981a) Selection pressures affecting male nutrient investment at mating in Heliconiine butterflies. Evolution 35:931–940
Boggs CL (1981b) Nutritional and life-history determinants of resource allocation in holometabolus insects. Am Nat 117:692–709
Boggs CL, Gilbert LE (1979) Male contribution to egg production in butterflies: evidence for transfer of nutrients at mating. Science 206:83–84
Boggs CL, Watt WB (1981) Population structure of pierid butterflies. IV. Genetic and physiological investment in off-spring by male Colias. Oecologia 50:320–324
Brakefield PM (1984) The ecological genetics of quantitative characters of Maniola jurtina and other butterflies. In: Vane-Wright RI, Ackery PR (eds) The biology of butterflies. Academic Press, London
Chew FS (1981) Coexistence and local extinction in two pierid butterflies. Am Nat 118:655–672
Drummond BA III (1984) Multiple mating and sperm competition in the Lepidoptera. In: Smith RL (ed) Sperm competition and the evolution of animal mating systems. Academic Press, New York
Elgar MA, Pierce NE (1988) Mating success and fecundity in an ant-tended Lycaenid butterfly. In: Clutton-Brock TH (ed) Reproductive success — studies of individual variation in contrasting breeding systems. University of Chicago Press, Chicago
Engebretson JA, Mason WH (1980) Transfer of 35Zn at mating in Heliothis virescent. Environ Entomol 9:119–121
Feltwell J (1982) Large white butterfly — The biology, biochemistry and physiology of Pieris brassicae. The Hague
Gilbert LE (1976) Postmating female odor in Heliconius butterflies: a male-contributed antiaphrodisiac. Science 193:419–420
Goss GJ (1977) The interaction between moths and pyrrolizidine alkaloid containing plants including nutrient transfer via the spermatophore in Lymire edwardsii (Ctenuchidae). PhD thesis, University of Miami, Miami, Florida
Greenfield MD (1982) The question of paternal investment in Lepidoptera: male-contributed proteins in Plodia interpunctella. Int J Invert Reproduc 5:323–330
Harcourt AH, Harvey PH, Larson SG, Short RV (1981) Testis weight, body weight and breeding system in primates. Nature 293:55–57
Harvey PH, Harcourt AH (1984) Sperm competition, testis size, and breeding systems in primates. In: Smith RL (ed) Sperm competition and the evolution of animal mating systems. Academic Press, New York
Jones KN, Odendaal FJ, Ehrlich PR (1986) Evidence against the spermatophore as paternal investment in the checkerspot butterflies (Euphydryas: Nymphalidae). Am Midl Nat 116:1–6
Lederhouse RC (1981) The effect of female mating frequency on egg fertility in the black swallowtail, Papilio polyxenes asterius (Papilionidae). J Lepid Soc 35:266–277
Marshall LD (1985) Protein and lipid composition of Colias philodice and C. eurytheme spermatophores and their changes over time. J Res Lepid 24:21–30
Parker GA (1970) Sperm competition and its evolutionary consequences in the insects. Biol Rev 45:525–567
Parker GA (1983) Arms races in evolution: An ESS to the opponent-independent costs game. J Theor Biol 101:619–648
Parker GA (1984) Sperm competition and the evolution of animal mating strategies. In: Smith RL (ed) Sperm competition and the evolution of animal mating systems. Academic Press, New York
Pliske TE (1973) Factors determining mating frequencies in some New World butterflies and skippers. Ann Entomol Soc Am 66:164–169
Pivnick KA, McNeil JN (1988) Puddling in butterflies. Physiol Ecol 12:461–472
Rutowski RL (1984) Sexual selection and the evolution of butterfly mating behavior. J Res Lepid 23:125–142
Rutowski RL, Gilchrist GW (1986) Copulation in Colias eurytheme (Lepidoptera: Pieridae): patterns and frequency. J Zool 209:115–124
Rutowski RL, Newton M, Schaefer J (1983) Interspecific variation in the size of the nutrient investment made by male butterflies during copulation. Evolution 37:708–713
Rutowski RL, Gilchrist GW, Terkanian B (1987) Female butterflies mated with recently mated males show reduced reproductive output. Behav Ecol Sociobiol 20:319–322
Scott JA (1972) Mating of butterflies. J Res Lepid 11:99–127
Scott JA (1986) The butterflies of North America: A natural history and field guide. Stanford University Press, Stanford, California
Sims SR (1979) Aspects of mating frequency and reproductive maturity in Papilio zelicaon. Am Midl Nat 102:36–50
Sugawara T (1979) Stretch reception in bursa copulatrix of the butterfly, Pieris rapae crucivora, and its role in behaviour. J Comp Physiol 130:191–199
Suzuki Y (1979) Mating frequency in females of the small cabbage white, Pieris rapae crucivora Boisduval (Lepidoptera, Pieridae). Kontyu 45:300–313
Svärd L, Wiklund C (1988a) Prolonged mating in the monarch butterfly Danaus plexippus and nightfall as a cue for sperm transfer. Oikos 51:351–354
Svärd L, Wiklund C (1988b) Fecundity, egg weight and longevity in relation to multiple matings in females of the monarch butterfly. Behav Ecol Sociobiol 23:39–43
Taylor OR (1967) Relationship of multiple mating to fertility in Atteva punctella (Lepidoptera: Yponomeutidae). Ann Entomol Soc Am 60:583–590
Thibout E (1975) Analyse des causes de l'inhibition de la récéptivité sexuelle et de l'influence d'un éventuelle second copulation sur la reproduction chez la Teigne du poireau, Acrolepia assectella (Lepidoptera: Pluttelidae). Entomol Exp Appl 18:105–116
Warren MS, Pollard E, Bibby TJ (1986) Annual and long-term changes in a population of the wood white butterfly Leptidea sinapis. J Anim Ecol 55:707–719
Watanabe M (1988) Multiple matings increase the fecundity of the yellow swallowtail butterfly, Papilio xuthus L., in summer generations. J Insect Behavior 1:17–30
Wickman P-O (1985) Territorial defence and mating success in males of the small heath butterfly Coenonympha pamphilus L. Anim Behav 33:1162–1168
Wickman P-O (1986) Courtship solicitation by females of the small heath butterfly, Coenonympha pamphilus (L.) (Lepidoptera: Satyridae) and their behaviour in relation to male territories before and after copulation. Anim Behav 34:153–157
Wickman P-O, Wiklund C (1983) Territorial defence and its seasonal decline in the speckled wood butterfly (Pararge aegeria). Anim Behav 31:1206–1216
Wiklund C (1982) Behavioural shift from courtship solicitation to mate avoidance in female ringlet butterflies (Aphantopus hyperanthus) after copulation. Anim Behav 30:790–793
Wiklund C, Forsberg J (1985) Courtship and the male discrimination between virgin and mated females in the orange tip butterfly Anthocharis cardamines. Anim Behav 34:328–332
Wiklund C, Karlsson B (1984) Egg size variation in satyrid butterflies: adaptive vs historical “Bauplan”, and mechanistic explanation. Oikos 43:391–400
Wiklund C, Persson A (1983) Fecundity, and the relation of egg weight variation to offspring fitness in the speckled wood butterfly Pararge aegeria, or why don't butterfly females lay more eggs? Oikos 40:53–63
Wiklund C, Karlsson B, Forsberg J (1987) Adaptive versus constraint explanation for egg-to-body size relationships in two butterfly families. Am Nat 130:828–838
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Svärd, L., Wiklund, C. Mass and production rate of ejaculates in relation to monandry/polyandry in butterflies. Behav Ecol Sociobiol 24, 395–402 (1989). https://doi.org/10.1007/BF00293267
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00293267