Abstract
Ventilation treatment of acute lung injury (ALI) requires the application of positive airway pressure at the end of expiration (PEEPapp) to avoid lung collapse. However, the total pressure exerted on the alveolar walls (PEEPtot) is the sum of PEEPapp and intrinsic PEEP (PEEPi), a hidden component. To measure PEEPtot, ventilation must be discontinued with an end-expiratory hold maneuver (EEHM). We hypothesized that artificial neural networks (ANN) could estimate the PEEPtot from flow and pressure tracings during ongoing mechanical ventilation. Ten pigs were mechanically ventilated, and the time constant of their respiratory system (τRS) was measured. We shortened their expiratory time (TE) according to multiples of τRS, obtaining different respiratory patterns (Rpat). Pressure (PAW) and flow (V′AW) at the airway opening during ongoing mechanical ventilation were simultaneously recorded, with and without the addition of external resistance. The last breath of each Rpat included an EEHM, which was used to compute the reference PEEPtot. The entire protocol was repeated after the induction of ALI with i.v. injection of oleic acid, and 382 tracings were obtained. The ANN had to extract the PEEPtot, from the tracings without an EEHM. ANN agreement with reference PEEPtot was assessed with the Bland–Altman method. Bland Altman analysis of estimation error by ANN showed −0.40 ± 2.84 (expressed as bias ± precision) and ±5.58 as limits of agreement (data expressed as cmH2O). The ANNs estimated the PEEPtot well at different levels of PEEPapp under dynamic conditions, opening up new possibilities in monitoring PEEPi in critically ill patients who require ventilator treatment.
Similar content being viewed by others
1 Introduction
Mechanical ventilation (MV) is currently used in patients with respiratory failure in the intensive care unit as well as during anesthesia in patients undergoing different surgical procedures. Caregivers, as part of respiratory therapy, may decide to maintain a certain amount of pressure in the lungs at the end of expiration in order to prevent end-expiratory lung collapse that may compromise oxygenation and gas exchange as well as make the lungs more prone to ventilation-induced lung injuries. This pressure is usually termed applied positive end-expiratory pressure (PEEPapp) and can be directly implemented on the control interface of the ventilator.
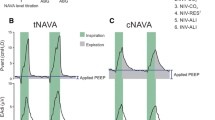
However, the total positive end-expiratory pressure (PEEPtot) inside the lungs at the end of expiration is generated by the combination of ventilator settings and the respiratory mechanics of the connected patient. The component of PEEPtot produced by incomplete expiration (Fig. 1) is termed intrinsic PEEP (PEEPi) [1]:
Respiratory tracings depicting the methods for detecting the persistence of alveolar pressure at the end of expiration. PEEP positive end expiratory pressure, PEEP app applied PEEP, PEEP i,dyn intrinsic PEEP measured in dynamic conditions, i.e. without stopping inspiratory flow, PEEP i,stat intrinsic PEEP measured in static conditions, i.e. during a breath-hold maneuver. Further explanation in the text
PEEPi develops as a result of two possible conditions: (a) a discrepancy between the allowed expiratory time and the time needed to empty the lungs according to the time constant (compliance and resistance) of the respiratory system and (b) the expiratory flow limitation, typical of chronic obstructive pulmonary disease and other pulmonary diseases where airways narrow and even close during expiration, trapping air.
During artificial ventilation, PEEPtot has positive and negative consequences for the respiratory and cardiovascular systems. The PEEPtot affects oxygenation, gas exchange (redistributing blood flow and ventilation) and respiratory system mechanics (shifting the pressure/volume curve of the lung). Furthermore, the PEEPtot has potential effects on the integrity of the lung parenchyma (increasing the potential for ventilator-induced lung injury) as well as cardiovascular dynamics (increasing the intra-thoracic pressure reduces venous return and cardiac output).
Thus, awareness of the PEEPtot is very important during mechanical ventilation. Although the PEEPapp is known to the physician, PEEPi is hidden and mostly unpredictable. Thus, it requires monitoring. Different methods have been proposed for estimating PEEPi during controlled mechanical ventilation (CMV). A frequently used method for estimating PEEPtot in paralyzed patients who are mechanically ventilated involves creating static conditions and applying an end-expiratory hold maneuver (EEHM), with cessation of gas flow at end-expiration from the airways. Once the PEEPtot and PEEPapp are known, PEEPi can be calculated (Eq. 1) [2, 3].
PEEPtot assessed with an EEHM is the reference value for patient monitoring. However, it requires the discontinuation of the cyclic succession of inspiration and expiration for a varying number of seconds, and in patients with acute respiratory distress, these repetitive respiratory pauses can be dangerous. Therefore, a continuous non-invasive monitoring method for PEEPtot would be advantageous.
We hypothesized that artificial neural networks (ANNs), fed by inspiratory airway pressure and flow, would enable the measurement of PEEPtot and its components PEEPi and PEEPapp during ongoing mechanical ventilation. The aim was to test this hypothesis in an animal model, training ANNs on examples of PEEPi generated by the progressive shortening of the expiratory time (TE).
Therefore, the objectives of the present contribution were as follows: to train one ANN to assess PEEPtot from dynamic respiratory tracings and to test the ANN performance, assessing any dependency of the estimation error by PEEPapp.
2 Materials and methods
2.1 Experimental design
This study was composed of two phases. During the first phase, a porcine model of pulmonary dynamic hyperinflation with the progressive reduction of the allowed expiratory time set on the ventilator was created. Pressure (PAW) and flow (V′AW) at the airway openings were recorded in different scenarios: with and without additional resistance, during healthy conditions and after acute lung injury. From these tracings, expiratory time constant (τRS), respiratory system resistance (RRS) and compliance (CRS), total static PEEP (PEEPtot,stat) and total dynamic PEEP (PEEPtot,dyn) were calculated. During the second phase, an artificial neural network was trained and tested to estimate PEEPtot,stat from the recorded signals.
2.2 Animal model
The present study was approved by the local institutional review board for the care of animal subjects; the care and the handling of the animals were in accordance with the regulations of the Swedish Board for Laboratory Animals and executed following the European Union Directive 2010/63/EU for animal experiments.
Ten healthy pigs (Hampshire, Yorkshire and Swedish mixed country breed; weight 30.0 ± 2.5 kg) were included in the study. They were premedicated with azaperone 40 mg im (Janssen, Wien, Austria) at the farm. Anesthesia was induced with intramuscular administration of atropine (0.04 mg/kg), tiletamine-zolazepam 5 mg/kg (Boehringer Ingelheim, Copenhagen, Denmark) and medetomidine 5 μg/kg (Orion Pharma AB, Sollentuna, Sweden). The trachea was intubated via tracheostomy, using a cuffed endotracheal tube (n. 5.5, Portex, Smiths Medical Inc, St Paul, USA). Then total intravenous anesthesia started, by the administration of ketamine 20 mg/kg/h (Vetpharma AB, Zurich, Switzerland), and fentanyl 5 μg/kg/h (Pharmalink, Spånga, Sweden). After adequate anesthesia was achieved (tested by lack of reaction to painful stimulation between the front hooves), i.v. infusion of pancuronium 0.24 mg/kg/h (Organon Teknika, Göteborg, Sweden) was added. During each experiment, the fluid replacement strategy, based on the constant infusion of Rehydrex® (Fresenius Kabi, Uppsala, Sweden) with glucose 25 mg/ml at a starting rate of 5 ml/kg/h, aimed at maintaining stable systemic arterial pressure. Instrumentation consisted of the insertion of an 18 gauge catheter into the left carotid artery for continuous invasive arterial pressure measurement and sampling of arterial blood gases. Another 18-gauge catheter was introduced into the right external jugular vein, together with a floating tip pulmonary artery (PA) catheter. The latter catheter was inserted and positioned as guided by the pressure tracings on the connected bedside monitor (CS/3™, Datex Ohmeda, Helsinki, Finland). This set-up allowed the measurement of cardiac output, with injection of cold boluses of physiological saline (approximately 10 ml per bolus at a temperature of 3–5°C, in triplicate) randomly during the respiratory cycle and the continuous monitoring of blood temperature by using the sensor located in the PA catheter. Urinary output was measured with a surgically inserted suprapubic bladder catheter. Arterial and mixed venous samples were taken to measure pO2, pCO2 and pH with a blood gas machine (ABL 300, Radiometer, Copenhagen, Denmark) during various phases of the experiment.
2.3 Ventilation and creation of intrinsic PEEP
At the beginning of each experimental session, all pigs were ventilated using volume controlled - constant flow mechanical ventilation (VC-MV; Servo 300, Siemens Elema, Solna, Sweden). The tidal volume (VT) was set between 8 and 9 ml/kg to obtain normocapnia (PaCO2 35–45 mm Hg). PEEPapp was initially set to 5 cmH2O. Inspiratory fraction of oxygen (FIO2) was maintained at 0.5. The inspiratory-to-expiratory ratio was set to 1:2 (s) and the respiratory rate 20 breaths/min. After a stabilization period of 45 min after instrumentation, the animals were randomized to receive an applied PEEP of 0, 5 or 10 cmH2O, and a baseline recording of airway pressure and flow (PAW and V′AW) was obtained. A tracing of the inspired volume (VRS) versus time was obtained from V′AW by integration. Using the slope of the expiratory volume/flow curve, the expiratory time constant of the respiratory system, τRS, was determined. This was used to calculate the ventilation patterns in the second part of the experiment. During constant minute ventilation and respiratory cycle time (TTOT), the expiratory time was progressively shortened in proportion to expiratory τRS. The experimental protocol was composed of different recording sessions of PAW and V′AW, characterized by an expiratory time equal to decreasing multiples of τRS, 3.0, 2.5, 2.0, 1.5, 1.0 times τRS. After the TE was set to a predefined multiple of τRS, ventilation was maintained until a steady state was reached (verified by observing a stable peak pressure) and never <5 min. Then PAW and V′AW were recorded for 10 or more consecutive cycles; the last breath had an end-inspiratory hold maneuver (EIHM) and an EEHM. This last breath was used to estimate PEEPtot,dyn and PEEPtot,stat.
After the measurements at the different multiples of τRS, the TE was returned to three times τRS, and resistance (RADD) was added in series with the endotracheal tube. It consisted of a pediatric n.3 tube (Mallinckrodt Medical, Athlone, Ireland). The purpose of its application was to increase tube resistance and to increase the time constant of the system connected to the ventilator. The same protocol of progressive reduction of the TE in multiples of τRS was applied in the presence of RADD. After the last recording, RADD was removed, and the TE was again returned to three times τRS.
Acute lung injury (ALI) was then induced by repeated injections of small boluses of oleic acid (OA; Apoteksbolaget, Göteborg, Sweden) into the central venous catheter, targeting a total dose of 0.1 ml/kg [4]. Administration of OA was suspended if SaO2 fell to 80 %. After a period of 2–3 h, when stable circulation had been achieved, similar recording sessions with a progressive reduction of the TE in proportion to τRS were obtained. Then, as before, the measurements were repeated with the additional resistance (RADD).
From the 10 animals involved in the study, a total of 20 recording sessions per animal were acquired in duplicate, for a total of 400 tracings. Of these, 18 of the 400 were discarded due to technical reasons, and the final pool of data included the remaining 382 tracings.
2.4 Recording of the respiratory variables
During mechanical ventilation, V′AW was recorded with a Fleisch pneumotachograph (Laminar Flow Element type PT, Special Instruments GmbH, Nördlingen, Germany) positioned between the endotracheal tube and the ventilator and connected to a differential pressure transducer (Diff-Cap Pressure Transducer, Special Instruments GmbH, Nördlingen, Germany). Calibration of the flow measurement device was performed by using a source of constant flow and a precision flow meter (Calibration Analyzer TS4121/P, Timeter Instrument Corporation, St. Louis, MO, USA) connected in series with the transducer to be calibrated.
PAW, esophageal and gastric pressures were measured with pressure transducers (Digima Clic Pressure Transducers, Special Instruments GmbH, Nördlingen, Germany). Signals from the transducers were forwarded to an analog/digital converter card (DAQ-card AI-16XE50, National Instruments Corp., Austin, TX, USA) and stored on a PC at a sampling frequency of 200 Hz, using BioBench software (ver. 1.0, National Instruments Corp.).
From the breath preceding the one used to feed the ANN, CRS and RRS were calculated by applying the interrupter technique, as described by Bates et al. [5].
2.5 Data analysis and the neural network
The tracings, after having been filtered in the range between 49.6 and 50.4 Hz, were downsampled at 100 Hz, in order to reduce redundancy among neighboring points. The onset of the inspiratory flow (tINIFLOW) was identified with a computer script: This was used to cut out from the tracing of V′AW and PAW the segments of curves extending from 0.1 s before tINIFLOW to 0.1 s after tINIFLOW (see Fig. 2). The segments (consisting of 20 V′AW points and 20 PAW points) formed an input pattern of 40 points that fed the neural network.
Data used to feed the artificial neural network (ANN). The input pattern was formed by 20 synchronous samples of PAW and V’ around the onset of the inspiratory flow. P AW pressure at the airway opening, V’ flow at airway opening, s seconds, t INIFLOW time of the onset of the inspiratory flow, PEEP tot total positive end expiratory pressure
The ANN was implemented via software on a computer (Neural Networks Toolbox ver. 3.0 for MatLab ver. 5, The MathWorks Inc., Natick, MA, USA). The learning algorithm was resilient backpropagation. The ANNs consisted of three layers, whose activating functions were log-sigmoids for the input and the intermediate layer and linear for the output layer. The number of neurons in the input layer was 40 (determined by the dimensions of the input pattern). The output layer consisted of 1 neuron, yielding PEEPtot,stat calculated by the ANN. To determine the best architecture, that is, the number of intermediate neurons that provided the best performance for the required task, we used eightfold cross-validation with early stopping. More details about this method can be found in the study by Haykin [6] and in our previous papers [7, 8]. The ANN architecture that showed the best performance had 18 intermediate neurons. The training data set included 305 input patterns out of 382 recordings; the remaining 77 were used as the data test set, to examine the performance of the ANN.
The ANN had to extract PEEPtot,stat from the input pattern described above. The ANN training consisted of giving at the same time for each input pattern the corresponding PEEPtot,stat, estimated by the EEHM. After training, the test phase consisted of feeding the ANN with input patterns not seen before and comparing the results it yielded to the PEEPtot,stat calculated separately.
We computed the linear regression between the results yielded by the ANN and the measure of PEEPtot,stat (by using the expiratory hold maneuver method) to assess the ANN’s performance on this task. We also analyzed the measurement error according to Bland and Altman [9, 10]. Plotting the ANN measurement error versus the applied PEEP and drawing a linear regression, we aimed at checking whether there was dependency between the two variables. We also analyzed the relation between PEEPtot,dyn and PEEPtot,stat in order to disclose any possible interdependence between the two variables that might influence the ANN’s performance.
3 Results
The time constant of the respiratory system in healthy conditions was 0.80 ± 0.13 s and was decreased to 0.74 ± 0.12 s after OA was administered. The mean RRS ranged from 16.80 ± 11.37 [cmH2O/(L/s)] (baseline) to 18.32 ± 18.27 [cmH2O/(L/s)] (with a TE of 1 τRS). Decreasing the expiratory time in proportion to τRS did not noticeably change RRS. The mean respiratory system compliance showed a tendency to decrease when the expiratory time was decreased. After ALI was induced with OA administration, at corresponding respiratory patterns, CRS was reduced, as expected. The main hemodynamic, respiratory and gas exchange variables are reported in Table 1.
Total PEEP, measured in dynamic conditions, tended to increase when the expiratory time was decreased. When total PEEP was measured in static conditions (Fig. 3), a similar tendency toward higher values for short expiratory times was found. The Bland–Altman analysis of the ANN estimation error (Fig. 4) showed −0.40 ± 2.84 (expressed as bias ± precision); thus, ±5.58 was the limit of agreement (data expressed as cmH2O). The linear regression of estimation error by ANN versus PEEPapp (Fig. 5) had an equation of y = 0.11 x −0.87 and the coefficient R = 0.1.
Different examples of PEEPtot,stat were created by shortening the expiratory time. They were assessed with the expiratory hold maneuver, which represents the gold standard for PEEP measurement. They were generated starting from different applied PEEP levels. Bars represent standard deviation. T EXP expiratory time expressed as multiple of expiratory time constants (τ), PEEP tot,stat total PEEP measured in static conditions, that is, during a breath-hold maneuver
The relation between PEEPtot,dyn and PEEPtot,stat is presented in Fig. 6: The coordinates of the 382 samples (corresponding to the training and testing data pools) are the values of PEEPtot,dyn (y-axis) versus PEEPtot,stat (x-axis) measured on the same tracing. It was expressed with the linear regression y = 0.25 x +1.11 and a R2 = 0.24.
Relation between PEEPtot,dyn (measured by the phase shift between the flow and pressure onsets) versus PEEPtot,stat (measured by the end-expiratory hold maneuver). The equation on the graph refers to the linear regression between the two variables. Of the 382 samples that are depicted in the figure, a minority of cases are positioned near the identity line. They represent conditions close to lung homogeneity, and have minimal PEEPi (measured as PEEPtot,dyn) similar to the mean PEEPi (measured as PEEPtot,stat) values
4 Discussion
We trained and tested one ANN to estimate PEEPtot,stat from pressure and flow tracings at airway openings during ongoing mechanical ventilation in an experimental porcine model. To create a pool of data to train the ANN, we used an animal model of dynamic pulmonary hyperinflation obtained by decreasing the expiratory time during mechanical ventilation. In our model, the ANN performance was excellent with a close correlation and low bias between the ANN and the standard method for obtaining PEEPtot,stat.
In a completely passive subject (i.e., muscle-paralyzed subject without spontaneous respiratory activity), expiration occurs passively because of the recoil of the elastic structures of the lung, previously stretched by the inspired volume of air. The proportion of the volume exhaled at the end of the expiration obeys the rules of compartmental kinetics:
where Vt is the volume contained in the lung at time t from the onset of expiration, V0 is the volume at the beginning of expiration and τRS is the time constant of the respiratory system. Although based on a simple mono-compartmental system, this model accounts for the rise in PEEPi when the expiration time is decreased, indicating an increase in the trapped volume. At the beginning of the experiment after instrumentation and after OA damage, we measured expiratory τRS. We chose to measure it during expiration because apparent τRS can be different during inspiration and expiration, due to variations in RRS and CRS at different lung volumes.
We aimed at generating PEEPi by decreasing the expiratory time in order to generate examples to train the ANN. We measured τRS from the slope of the expiratory volume/flow curve as described by McIlroy et al. [11]. This kind of analysis, based on a mono-compartmental model, cannot detect the mechanical behavior generated by additional compartments (in lung inhomogeneity) or by mechanical nonlinearities as in the presence of marked “flow limitation” typical of patients with chronic obstructive lung disease [12]. However, for our purposes, we considered the following method adequate: if the respiratory system subtended “longer” time constants (because of neglected obstructive or viscoelastic components), generation of PEEPi by shortening the expiratory time could have been enhanced. Moreover, the mono-compartmental model was not transferred to the ANN, which had to learn how to estimate PEEPtot through examples.
We decided to study ANNs during lung injury due to the need to estimate their performances in non-healthy conditions. Several models of lung injury have been described in the literature, and each model has its own peculiarities in terms of hemodynamics and respiratory mechanics [4]. We arbitrarily chose to use a model based on the intravenous administration of oleic acid that mimics the early phase of adult respiratory distress syndrome (ARDS) by developing important alterations of lung permeability and by releasing inflammatory mediators. In principle, the study of a single, specific kind of lung injury can represent a limit to the general applicability of the ANN method. Thus, in the near future, it may be advisable to test the ANN performance on other models of lung disease.
Intrinsic PEEP can be measured in static or dynamic conditions. Static measurements require an EEHM. The different mechanical compartments of the lung can retain a variable amount of gas at the end of the expiration, in relation to the expiratory time constant [13]. EEHM allows the equilibration of pressures between these compartments and the airway conduction system up to the pressure transducer. This value represents the weighted mean of the pressures inside the lung compartments at end expiration (regional PEEPi) and may include the contribution of the elasticity of the tubing system.
Dynamic measurement of PEEPi in completely passive mechanically ventilated subjects does not involve an end-expiratory pause. In the presence of PEEPi, the rise in airway pressure precedes the rise of flow. At the end of the preceding expiration, the lungs have retained a volume of gas that generates PEEPi. To force the gas flow to enter the lung during the following inspiration, a gradient of pressure must be created between the airways and the peripheral compartments. Therefore, the flow starts only when the pressure applied to the airways exceeds PEEPi [3].
If the lung has compartments that have different time constants, PEEPi measured with this method represents the minimum regional PEEPi, that is, the minimum pressure the ventilator has to generate in order to make air enter the lung [2, 13]. If the lung is perfectly homogenous, PEEPi measured with the static and dynamic methods is the same. However, this homogeneity cannot be observed even in physiological conditions. These two measurements reflect the relation between the minimum and average PEEPi so closely that the ratio between dynamic and static PEEPi has been used as a measure of lung homogeneity [14] and defined as the inequality index. According to Maltais and coworkers, the fewer the obstructive components of the lungs (a sign of inhomogeneous pathology), the closer the PEEPi,dyn and PEEPi,stat values.
When external PEEP is applied, measurement in either static or dynamic conditions yields PEEPtot, that is, the sum of the effects of PEEPi and PEEPapp. To separate these effects, Eq. 1 can be applied.
The ANN used in this paper was trained and tested according to accepted standards and based on our previous studies [6–8, 15]. As confirmed by the computer science literature, ANNs are universal function approximators [16, 17]. We decided to test their application to the estimation of PEEPtot during mechanical ventilation. Other studies have addressed the problem of monitoring PEEPi online. Eberhard et al. [18] applied a technique based on the least squares fitting method on a breath-by-breath basis, tested in patients who presented with acute respiratory distress syndrome. Nucci et al. [19] studied in a group of eight patients the performance of the recursive least squares method to obtain PEEPi online and stable and repeatable results. More recently, Mayaud et al. [20] developed an expert system for calculating a series of respiratory parameters that included PEEPi. However, they analyzed 23 tracings from patients who were breathing spontaneously, and not all presented a detectable PEEPi. Although the researchers found good agreement between their algorithm and the manual computation of PEEPi, only one case had a PEEPi higher than 5 cmH2O. Thus, the performance of their method must be validated in a wider range of conditions.
The ANN described in the present paper shares with other multilayer perceptrons the limitation derived from the choice of the pool of training examples. Although having the capacity of generalizing its knowledge, if the ANN has to face completely different patterns during use, similar to the patterns derived from other ventilation modalities or different pathologies, the ANN must be retrained on tracings that contain the new examples. The same limitations apply to the mechanical implications of muscle relaxation used in the present protocol. If the ANN has to face tracings derived from a spontaneously breathing subject, it must be retrained. Artificial neural networks process data using a complex matrix of connections between the artificial neurons. This implies that ANNs act similarly to a black box in which identification of the algorithm behind their performance is very difficult. From the clinical point of view, this will not show whether the calculation of PEEPtot, stat is based on an estimate of the inhomogeneity in PEEPi or the elastic components of the respiratory system.
In the first step, the experimental model was used to produce a pool of examples of PEEPtot, necessary for training the ANN. The shape of PEEPtot,stat plotted versus TE (Fig. 3) showed an exponential pattern, as expected by the respiratory dynamics. The increase in PEEP and in the subsequently trapped volume increased exponentially at linear decreases in the TE.
A Bland–Altman test showed low bias and scatter of the ANN estimate of PEEPtot,stat. Moreover, evaluation of the dependency of the estimation error by the ANN and applied PEEP demonstrated the absence of such a correlation. Thus, we concluded that the ANN could estimate PEEPtot,stat reliably, without any influence from the level of PEEPapp. To obtain PEEPi from PEEPtot, it is sufficient to subtract PEEPapp (which can be easily read on the ventilator display). Another issue we wanted to disclose was the possibility that the ANN would have approximated PEEPtot,stat (by using dynamic tracings) because of a potential strict correlation between PEEPtot,dyn and PEEPtot,stat. Our data showed that it was not the case, because, in our pool of data, only the 24 % of the PEEPtot,dyn values could be inferred by the corresponding PEEPtot,stat (see Fig. 6). This is in line with the concept that PEEPtot,dyn approximates PEEPtot,stat more closely in situations of pulmonary homogeneity [21], and we observed this in our experiments in a healthy lung before we administered the oleic acid. Comparison of the PEEPtot estimate by the ANN with the manually computed PEEPtot,stat and PEEPtot,dyn (see Fig. 7) supported the finding that the ANN yielded results that were directly related to PEEPtot,stat.
Plot comparing the manual measurements of PEEPtot,stat and PEEPtot,dyn with the measurement of PEEPtot made by the ANN on the same tracing. On the y-axis: estimation of PEEPtot with the three methods. Tracings (on the x-axis) are ordered according to increasing values of PEEPtot,stat, from data set 1 to 77. PEEPtot,stat (reference measure) was assessed with the end-expiratory hold maneuver; PEEPtot,dyn was calculated by the shift between the onset of inspiratory flow and pressure. The ANN estimate follows the PEEPtot,stat more closely than the PEEPtot,dyn
The ANN that had been taught with PEEPtot,stat showed good agreement with the manually calculated PEEPtot,stat, although the flow and pressure recordings in the selected regions are commonly used to calculate PEEPtot,dyn. This suggests that more information is available in the PAW and V’ curves than in the phase shift between them that is used for the manual calculation of PEEPtot,dyn.
To measure PEEPtot,stat for research purposes, application of EEHM remains the gold standard of reference, and for precision. However, when the purpose is the continuous monitoring of a variable, the measurement property of measure that should be aimed is robustness. In this respect, although this paper was not designed to assess the robustness of ANNs in continuous monitoring of PEEPtot,stat, this property of multilayer perceptrons is well demonstrated in the literature of this field [22].
Whether the reported limit of agreement (where 95 % of the measurement errors are positioned) is acceptable for a monitoring method should be discussed. In the present experiment, we measured the direct estimation of total PEEPtot,stat on single tracings, “not seen before” by the ANN and without any averaging of the computation over the presentation of different tracings on the same condition. We can expect that an ANN that faces recursively different breaths of the same tracing during ongoing ventilation would minimize the random error, because the estimation error is averaged over the elaboration of different tracings. Nevertheless, these theoretical speculations require a purposely designed study, in which the limits of precision of routine use are assessed including repetitive presentation of the same trace and adding a comparison with other accepted standards of monitoring PEEPi, such as the multilinear fitting and the use of phase shift between pressure and flow.
Intrinsic PEEP has been a frequently debated topic of critical care for many years [23]. The importance of PEEPi derives from the physiological consequences of its presence, spanning from alteration of hemodynamics (mainly the reduction of cardiac output due to a decrease in cardiac filling caused by the positive intrathoracic pressure) [24] to the potential risk of barotrauma (due to the possible high alveolar pressures) [2].
Moreover, as first reported by Jonson et al. [25], if PEEPi is not considered, a significant error can affect the measurement of static CRS. This finding led researchers to introduce this correction factor when estimating respiratory mechanics [26–28]. In previous studies, we demonstrated the capability of an ANN to extract information from respiratory signals [7, 8, 29]. Another mechanical consequence of PEEPi is the generation of higher levels of lung strain [30, 31], which, as result of pulmonary inhomogeneity, may induce a ventilator-induced lung injury [32]. The possibility of estimated PEEPtot,stat during ongoing mechanical ventilation is an important achievement. PEEPi measured by applying an end-expiratory pause requires a breath hold that must be prolonged by more than 1 s, and often more than 3 s in order to display a stable PEEPi value [33]. Moreover, in conditions of pulmonary inhomogeneity, occlusion of up to 5 s may be required [34].
The potential for hemodynamic and gas exchange impairment that these conditions present may not allow frequent and prolonged alterations of the breathing pattern. The estimation of PEEPtot by ANNs does not require the cessation of the breath series. This opens up a perspective in the field of respiratory mechanics monitoring, because of the possibility of estimating PEEPi in safer conditions. Further studies are necessary to ascertain that the performance shown by the ANN in the assessment of PEEPi in this experimental setting can be reproduced in different models of lung pathology and during different ventilatory strategies.
References
Rossi A, Polese G, Milic-Emili J. Monitoring respiratory mechanics in ventilator dependent patients. In: Tobin MJ, editor. Principles and practice of intensive care monitoring. New York: McGraw-Hill; 1998. p. 553–97.
Rossi A, Polese G, Brandi G, Conti G. Intrinsic positive end-expiratory pressure (PEEPi). Intensive Care Med. 1995;21:522–36.
Brochard L. Intrinsic (or auto-)PEEP during controlled mechanical ventilation. Intensive Care Med. 2002;28:1376–8.
Matute-Bello G, Frevert CW, Martin TR. Animal model of acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2008;295:379–99.
Bates JHT, Rossi A, Milic-Emili J. Analysis of the behavior of the respiratory system with constant inspiratory flow. J Appl Physiol. 1985;58:1840–8.
Haykin S. Cross-validation. In: Haykin S, editor. Neural networks a comprehensive foundation. London: Prentice-Hall International; 1999. p. 213–8.
Perchiazzi G, Högman M, Rylander C, Giuliani R, Fiore T, Hedenstierna G, et al. Assessment of respiratory system mechanics by artificial neural networks: an exploratory study. J Appl Physiol. 2001;90:1817–24. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11299272
Perchiazzi G, Giuliani R, Ruggiero L, Fiore T, Hedenstierna G. Estimating respiratory system compliance during mechanical ventilation using artificial neural networks. Anesth Analg. 2003;97:1143–8.
Altman GD, Bland JM. Measurement in medicine: the analysis of method comparison studies. Statistician. 1983;32:307–17.
Bland MJ, Altman GD. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–10.
McIlroy MB, Tierney DF, Nadel JA. A new method for measurement of compliance and resistance of lungs and thorax. J Appl Physiol. 1963;18:424–7.
Peslin R, Felicio da Silva J, Chabot F, Duvivier C. Respiratory mechanics studied by multiple linear regression in unsedated ventilated patients. Eur Respir J. 1992;5:871–8.
Laghi F, Goyal A. Auto-PEEP in respiratory failure. Minerva Anestesiol. 2012;78:201–21.
Maltais F, Reissmann H, Navalesi P, Hernandez P, Gursahaney A, Ranieri VM, Sovilj M, Gottfried S. Comparison of static and dynamic measurements of intrinsic PEEP in mechanically ventilated patients. Am J Respir Crit Care Med. 1994;150:1318–24.
Haykin S. Heuristics for making the back-propagation algorithm perform better. In: Haykin S, editor. Neural networks, a comprehensive foundation. London: Prentice-Hall International; 1999. p. 178–84.
Haykin S. Approximation of functions: universal approximation theorem. In: Haykin S, editor. Neural networks a comprehensive foundation. London: Prentice-Hall International; 1999. p. 208–9.
Castro LJ, Mantas JC, Benitez MJ. Neural networks with a continuous squashing function in the output are universal approximators. Neural Netw. 2000;13:561–3.
Guttmann J, Eberhard L, Wolff G, Bertschmann W, Zeravik J, Adolph M. Maneuver-free determination of compliance and resistance in ventilated ARDS patients. Chest J. 1992;102:1235–42.
Nucci G, Mergoni M, Bricchi C, Polese G, Cobelli C, Rossi A. O line monitoring of intrinsic PEEP in ventilator-dependent patients. J Appl Physiol. 2000;89:985–95.
Mayaud L, Lejaille M, Prigent H, Louis B, Fauroux B, Lofaso F. An open-source software for automatic calculation of respiratory parameters based on esophageal pressure. Respir Physiol Neurobiol. 2014;192:1–6. doi:10.1016/j.resp.2013.11.007.
Appendini L. About a relevance of dynamic intrinsic PEEP (PEEPi, dyn) measurement. Intensive Care Med. 1999;25:254.
Kerlirzin P, Vallet F. Robustness in multilayer perceptrons. Neural Comput. 1993;5:473–82.
Antonaglia V, Ferluga M, Capitanio G, Lucangelo U, Piller F, Roman-Pognuz E, et al. Respiratory mechanics in COPD patients who failed non-invasive ventilation: role of intrinsic PEEP. Respir Physiol Neurobiol. 2012;184:35–40. doi:10.1016/j.resp.2012.07.009.
Berlin D. Hemodynamic consequences of auto-PEEP. J Intensive Care Med. 2012;29:81–6.
Jonson B, Nordstrom L, Olsson SG, Akerback D. Monitoring of ventilation and lung mechanics during automatic ventilation. A new device. Bull Physiopathol Respir. 1975;11:729–43.
Rossi A, Gottfried SB, Zocchi L, Higgs BD, Lennox S, Calverley PAM, et al. Measurement of static compliance of the total respiratory system in patients with acute respiratory failure during mechanical ventilation. The effect of intrinsic positive end-expiratory pressure. Am Rev Respir Dis. 1985;131:672–7.
Bernasconi M, Ploysongsang Y, Gottfried SB, Milic-Emili J, Rossi A. Respiratory compliance and resistance in mechanically ventilated patients with acute respiratory failure. Intensive Care Med. 1998;14:547–53.
Broseghini C, Brandolese R, Poggi R, Bernasconi M, Manzin E, Rossi A. Respiratory resistance and intrinsic positive end-expiratory pressure (PEEPi) in patients with the adult respiratory distress syndrome (ARDS). Eur Respir J. 1988;1:726–31.
Perchiazzi G, Giuliani R, Vena A, Giustolisi O, Vetruccio T, Fiore T, et al. Robustness of two methods for estimating respiratory system compliance during mechanical ventilation. Intensive Care Med. 2003;29:168.
Chiumello D, Carlesso E, Cadringher P, Caironi P, Valenza F, Polli F, et al. Lung stress and strain during mechanical ventilation of the acute respiratory distress syndrome. Am J Respir Crit Care Med. 2008;178:346–55.
Perchiazzi G, Rylander C, Vena A, Derosa S, Polieri D, Fiore T, et al. Lung regional stress and strain as a function of posture and ventilatory mode. J Appl Physiol. 2011;110:1374–83.
Slutsky AS. Lung injury caused by mechanical ventilation. Chest J. 1999;116:9S–15S.
Pardaens PK, Van de Woestijne PK, Clement J. A physical model of expiration. J Appl Physiol. 1972;33:479–90.
D’Angelo E, Calderini E, Torri G, Robatto FM, Bono D, et al. Respiratory mechanics in anesthetized paralyzed humans: effects of flow, volume and time. J Appl Physiol. 1989;67:2556–64.
Acknowledgments
This study was supported by grants from the Swedish Medical Research Council (5315, K2015-99X-22731-01-04); the Swedish Heart-Lung Fund; the School of Anesthesiology and Intensive Care Medicine, Bari University, Italy; and the Center of Innovative Technologies for Signal Detection and Processing (TIRES), Bari University, Italy. The authors wish to thank Karin Fagerbrink, Eva-Maria Hedin and Agneta Roneus, laboratory technicians in the Department of Clinical Physiology, Uppsala University, for their support during the studies. Special thanks to Sergio Santostasi, engineering designer and developer of the data acquisition system, for his constant assistance during the experiments.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national and institutional guidelines for the care and use of animals were followed.
Additional information
The study was performed at the Hedenstierna Laboratory, University Hospital, Uppsala, Sweden.
Rights and permissions
About this article
Cite this article
Perchiazzi, G., Rylander, C., Pellegrini, M. et al. Monitoring of total positive end-expiratory pressure during mechanical ventilation by artificial neural networks. J Clin Monit Comput 31, 551–559 (2017). https://doi.org/10.1007/s10877-016-9874-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10877-016-9874-0